Reaction Rates
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
What is collision theory?
A reaction won’t take place between two particles unless they collide in the right direction with sufficient energy
What is the activation energy?
The minimum amount of kinetic energy particles needed to react
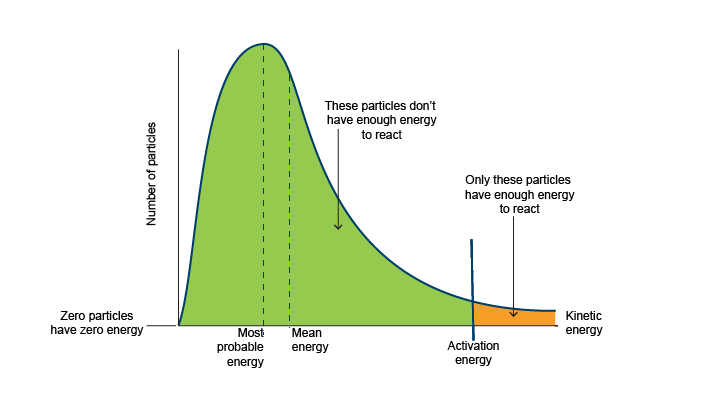
What is a Maxwell-Boltzmann distribution?
If you plot a graph of the numbers of molecules in a gas with different kinetic energies you get a Maxwell-Boltzmann distribution
This is a theoretical model that has been developed to explain scientific observations
The area under the curve is equal to the total number of molecules
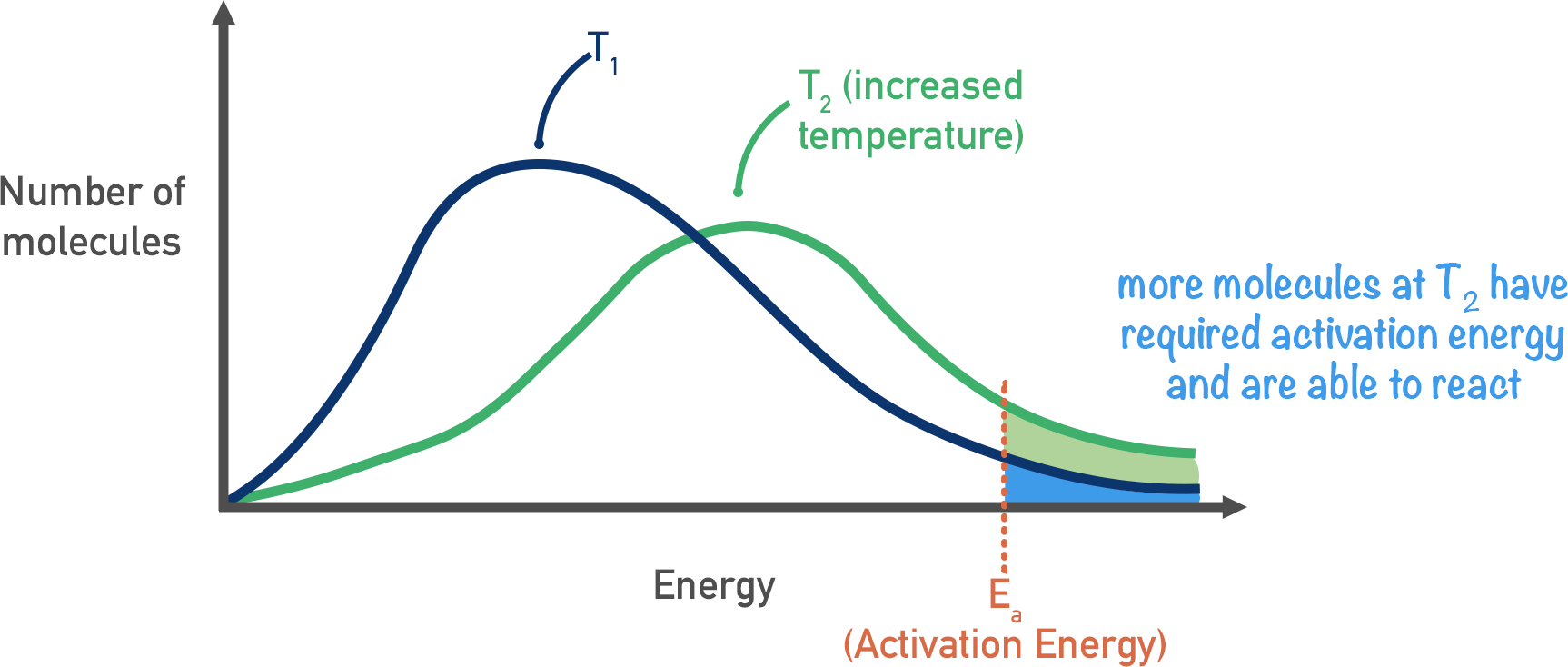
What is the effect of temperature on reaction rate?
When you increase temperature, the molecules will have more kinetic energy and will move faster.
More molecules would have the activation energy and be able to react
The maxwell-boltzmann curve shifts to the right- the area under the curve is still the same
Also, when kinetic energy is higher, there are more frequent collisions so reaction rate increases.
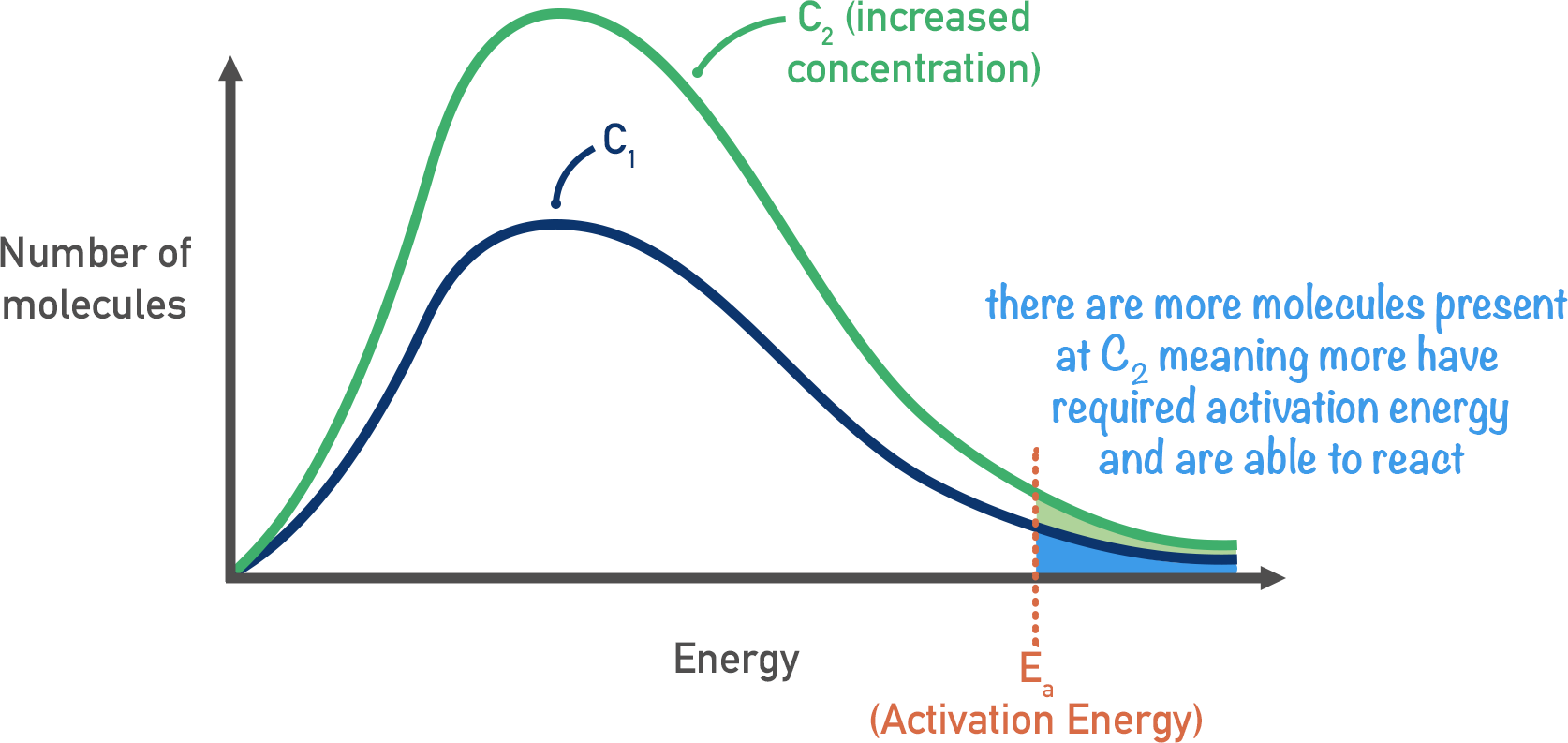
What is the effect of concentration on reaction rate?
If you increase the concentration of reactants in a solution, the particles will be closer together. If they are closer, collision are more frequent which increases rate of reaction
If the reaction is gaseous, increasing pressure has the same effect in a given volume