Synthetic Transformations
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
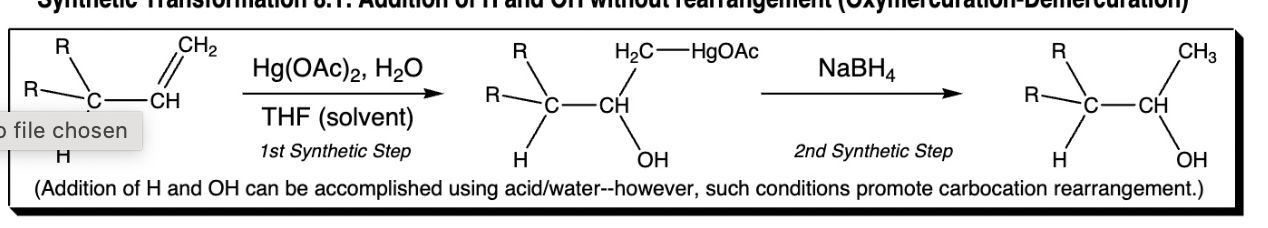
addition of H and OH without rearangement
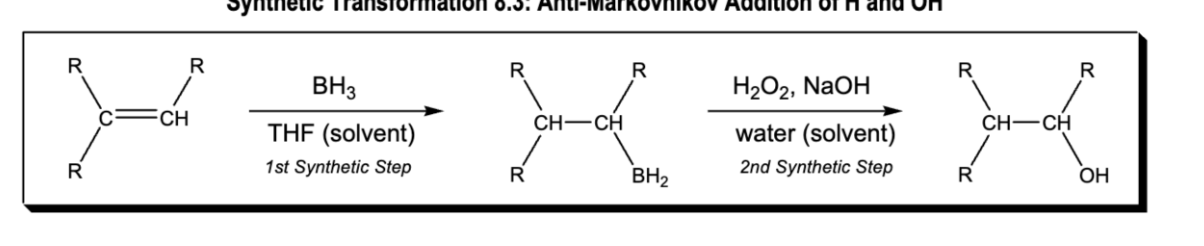
Anti mark of H and OH
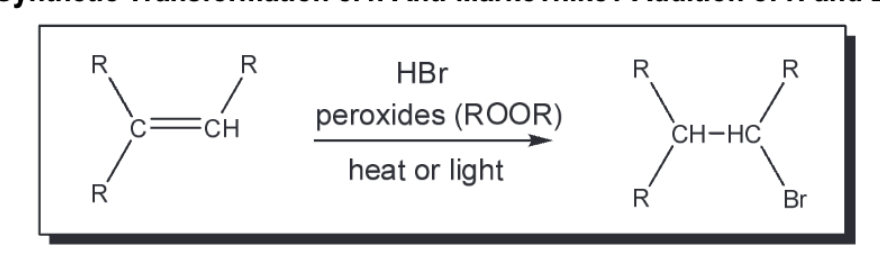
Anti Mark of H and Br
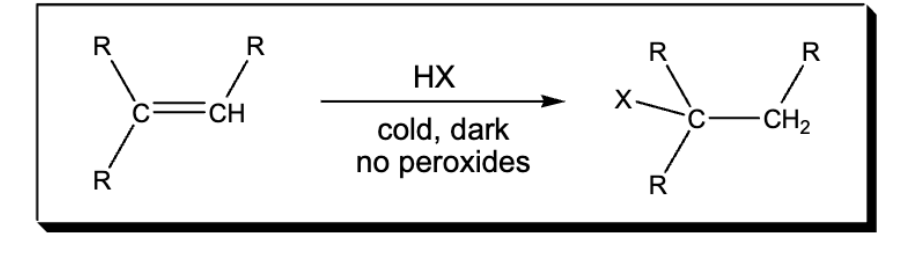
Markovnikov addition of H and X
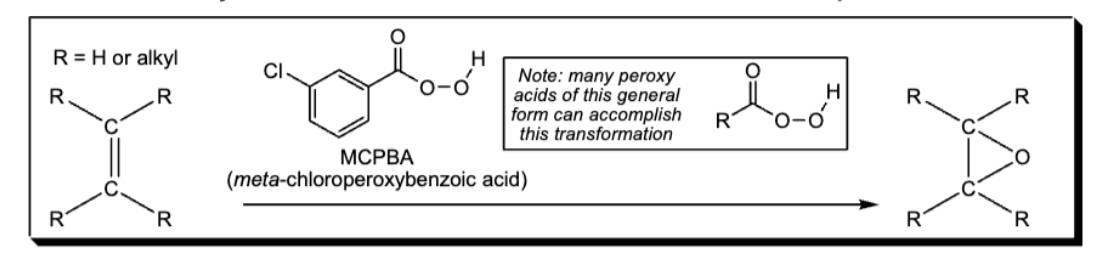
Oxidation of Alkene to epoxide
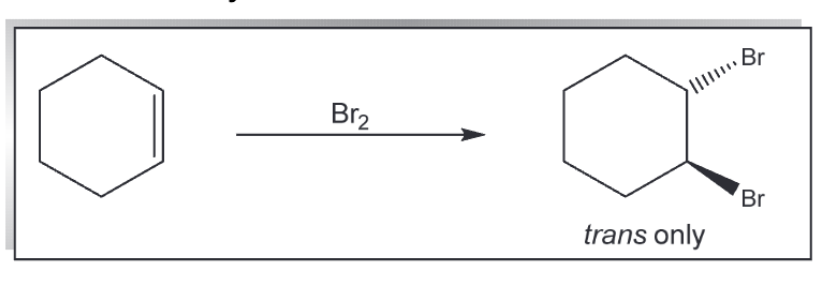
addition of Br2 to an alkene
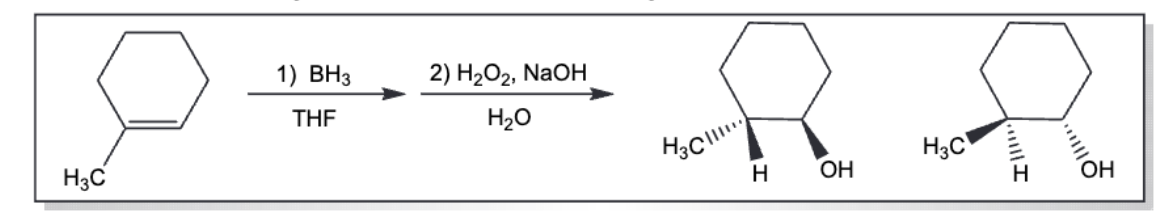
anti markovnikov epoxide ring opening (basic)
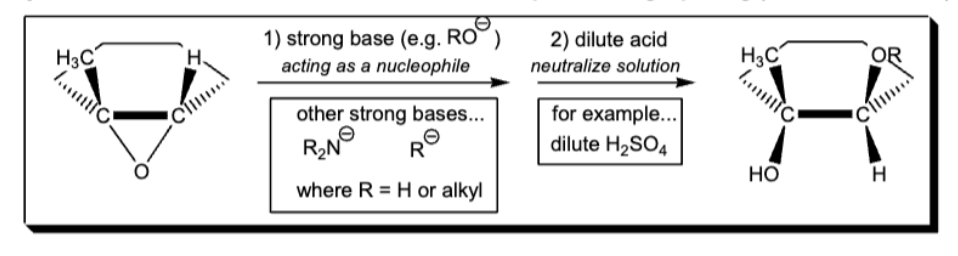
mark epoxide ring opening (acidic)
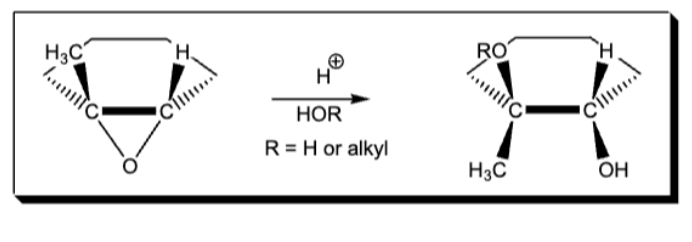
Markov addition of Br and OR to aleke
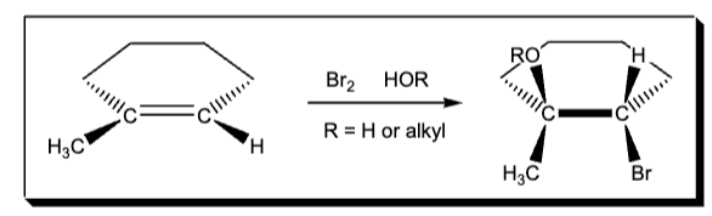
hydrocarbon oxidation X
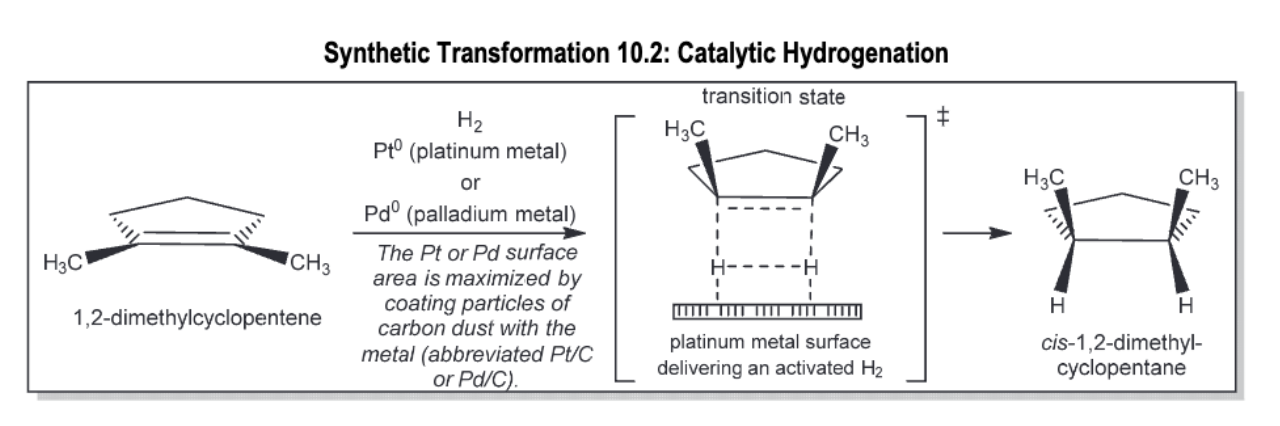
Catalytic hydrogenation
Stereoselective Alkyne Reductions (triple to single)

stereoselective alkyne reductions (triple to double e)
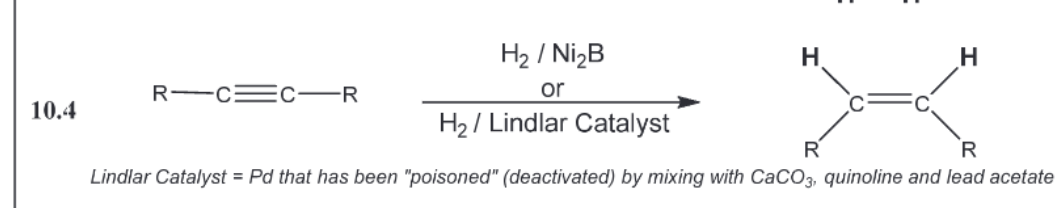
stereoselective alkyne reductnons (triple to single z)
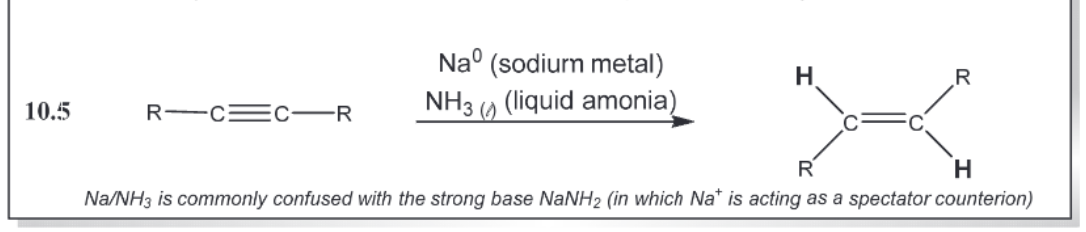
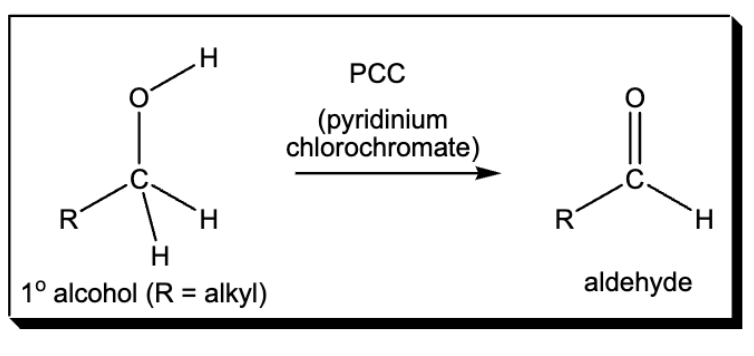
oxidation of 1 alcohol to aldehyde
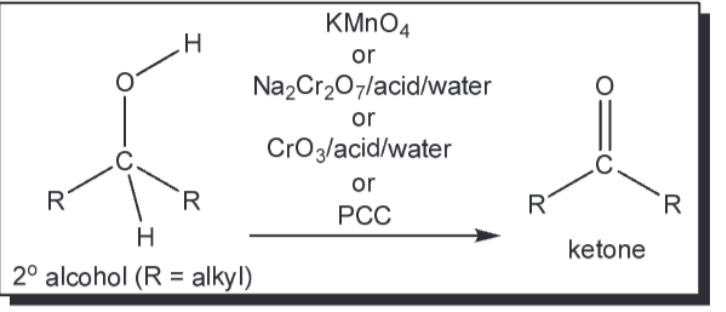
oxidation of 2 alcohol to ketone
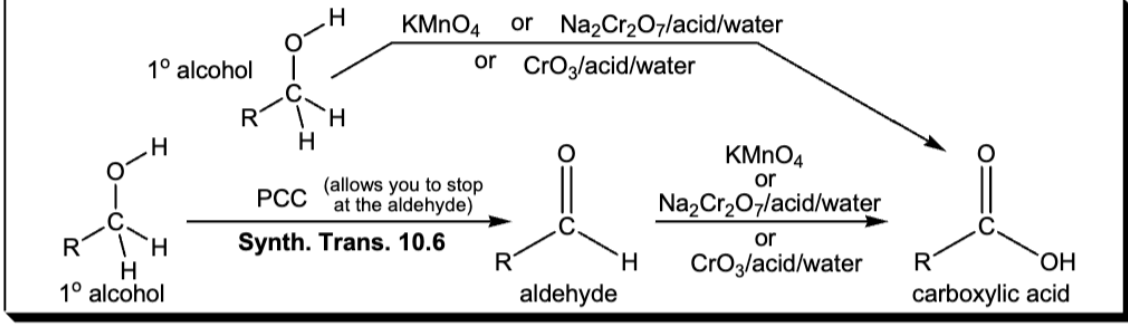
oxidation of a 1 alcohol or aldehyde to a carboxlyic acid
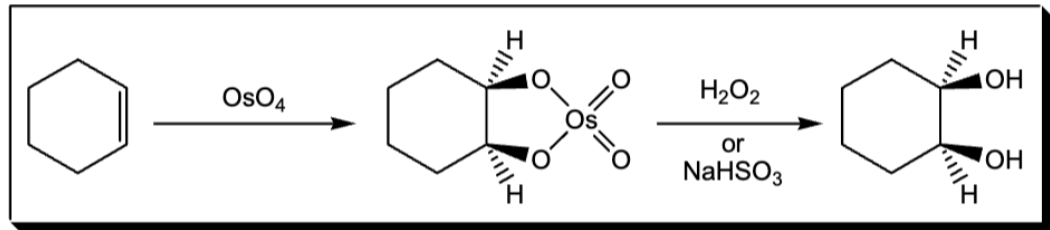
syn addition of two Oh groups to a pi bond
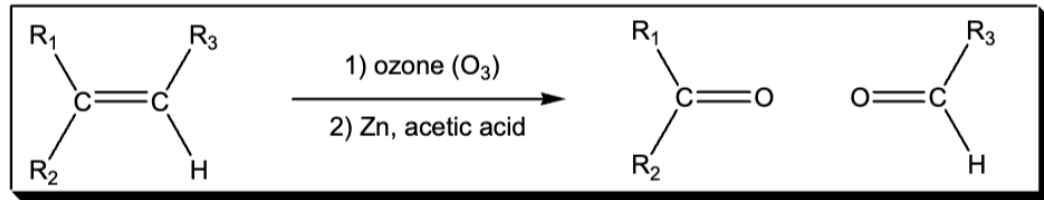
cleavage of a cc double bond using ozone
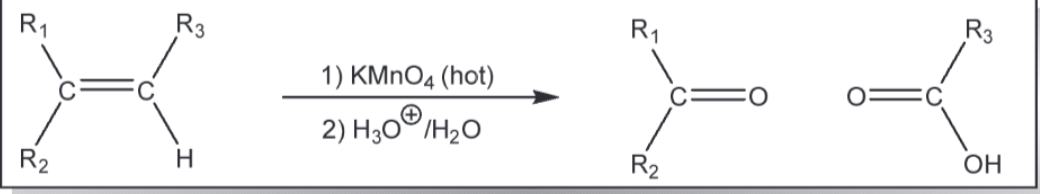
cleavage of a cc double bond using permannganate
markovnikov addition of H and X
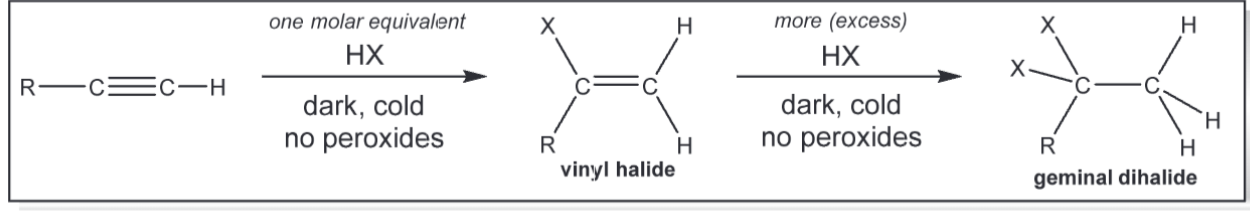
addition of X2 (x = cl or br)
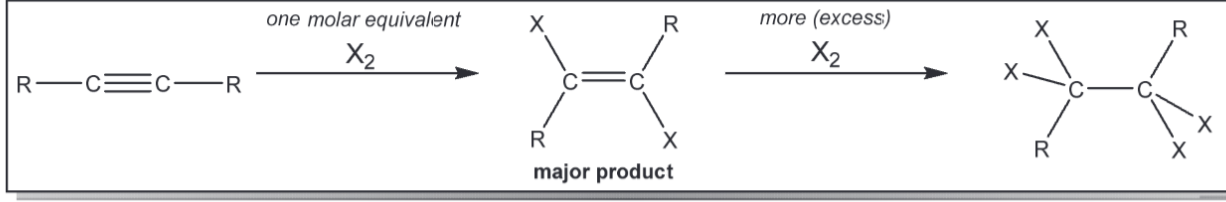
markov additio nof H2o to an Alkyne

anti markov addition of H20 to an alkyne
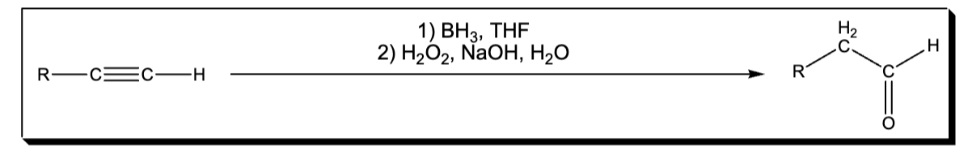
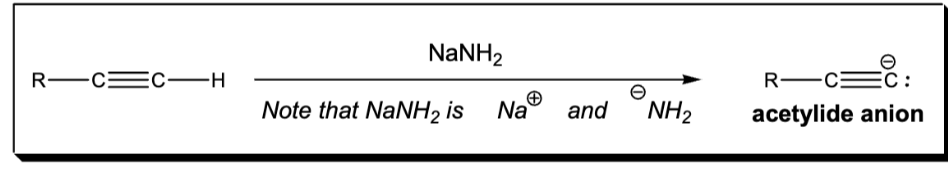
deprotonation of an alkyne
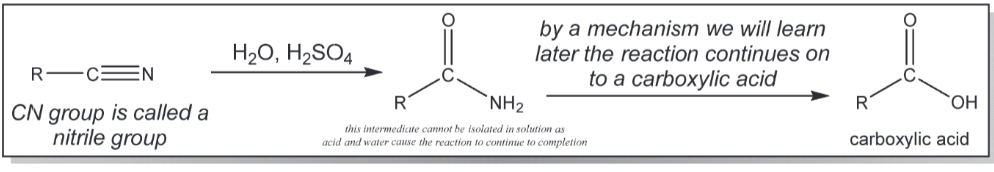
nitrile hydrolysis
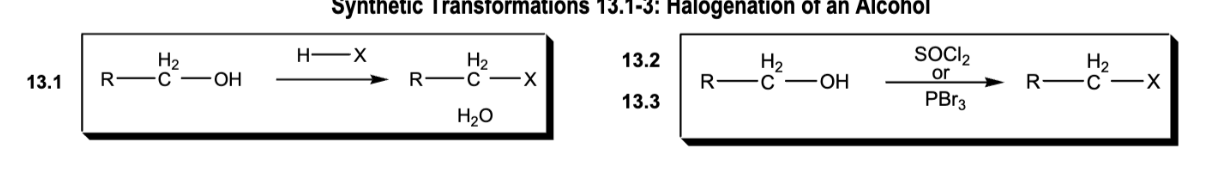
Halogenation of an alcohol
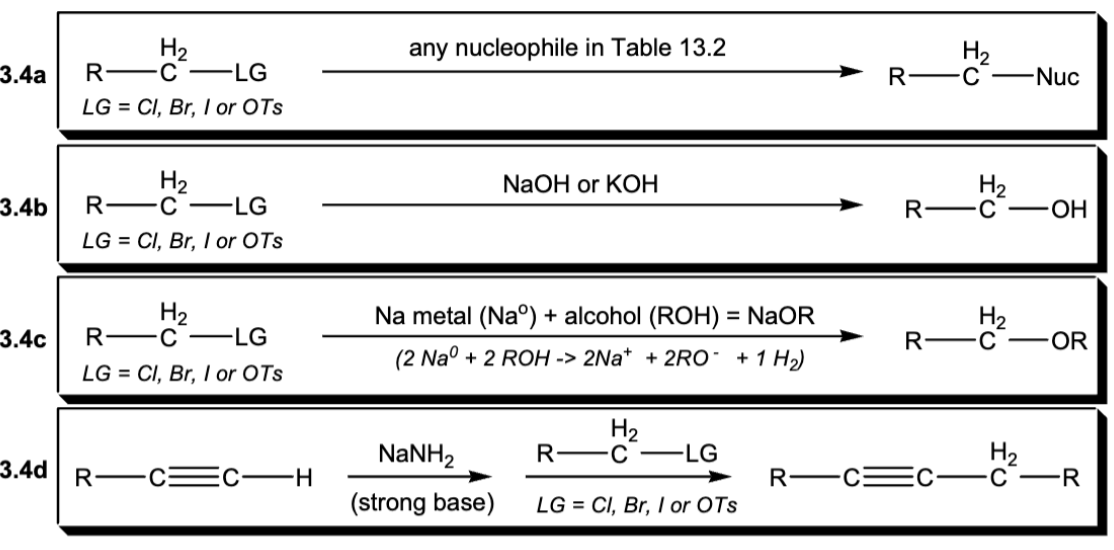
Examples of synthetic uses of Sn2 reactions