equilibrium
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
what is a chemical system
The chemicals involved in a reaction form the chemical system. Anything around it forms the surroundings.
what is a closed system
If the chemicals are contained within a space and cannot enter or leave, the system is a closed system.
what is an open system
If substances can be added or lost, the system is an open system.
what is physical change
products change state and physical properties alter. (Example: boiling water to form steam.)
what is chemical change
reactants form products with different physical and chemical properties.
what are reversible reactions
reactions where reactants form products and products form reactants.
All physical changes are reversible. Only some chemical changes are reversible.
A double arrow is used to show that a reaction can occur in both directions:
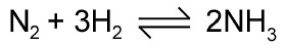
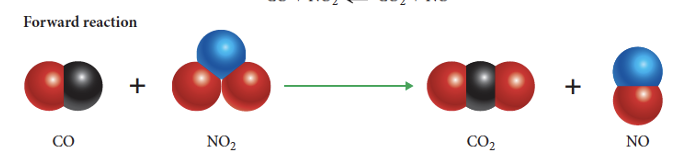
what is the forward reaction
reactants forming products
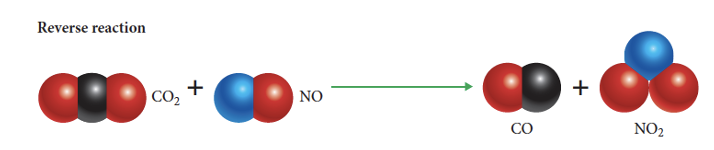
what is the reverse reaction
products forming reactants
The reversibility of chemical reactions is dependent on
the activation energies of both the forward and reverse reactions.
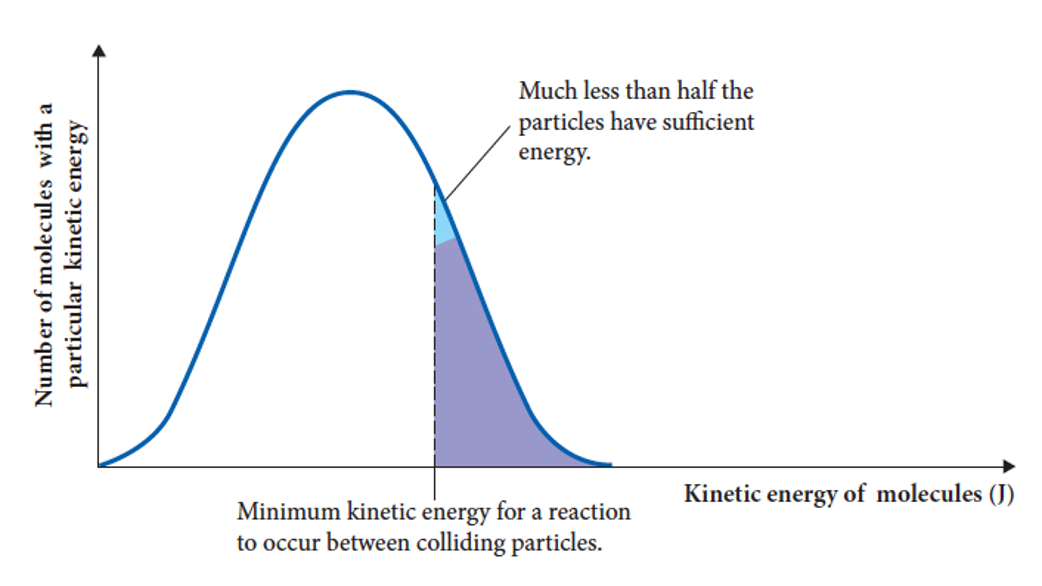
what is activation energy
the amount of energy required to break the bonds of the reactants
it is the difference in enthalpy between reactants and activated complex
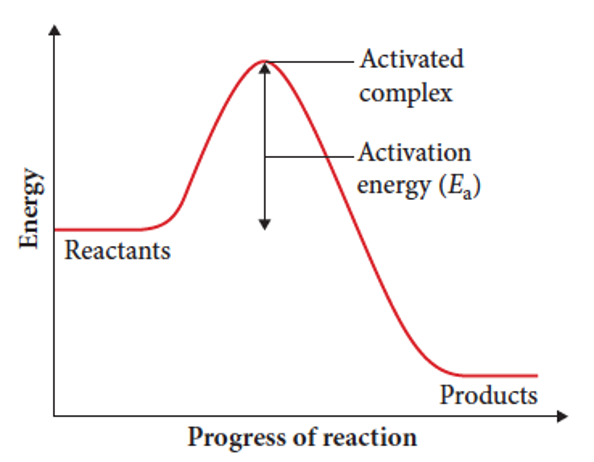
when are reversible reactions likely to form
when the activation energies of both forward and reverse reactions are low enough that sufficient particles have energy for a successful collision
what is dynamic equilibrium
the state reached when amount/concentration of reactants and products remain constant
the rates of forward and reverse reactions are equal
what happens when you act reactants to a chemical system
it results in a fast initial rate of forward reaction that gradually slows as reactants form products. the rate of the forward reaction slows and the rate of the reverse reaction increases as concentrations change.
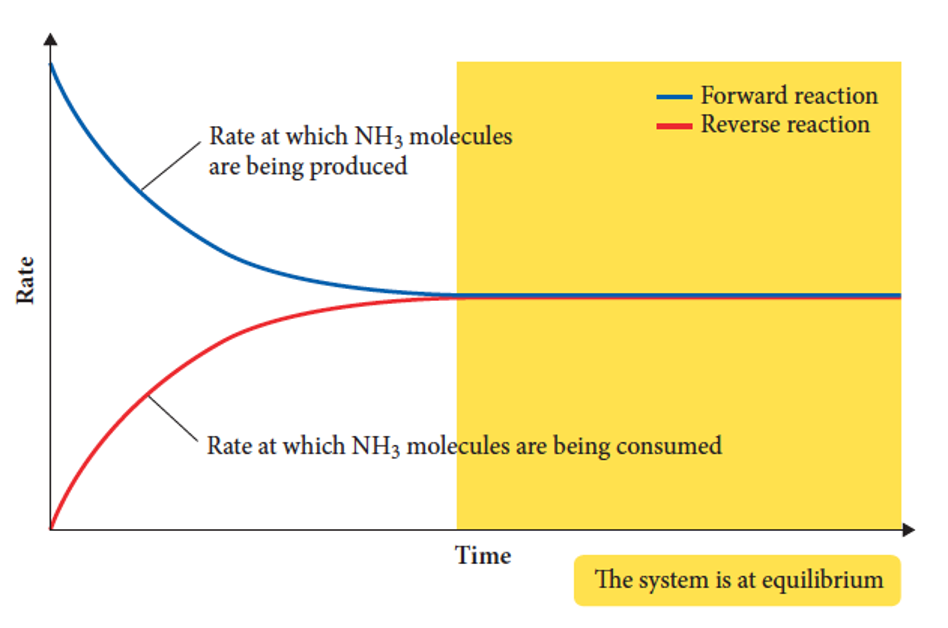
when does dynamic equilibrium happen
when the rates of forward and reverse reactions are equal and concentrations of products and reactants are constant.
summarise this
A chemical system will reach equilibrium if it:
•is a closed system
•involves a reversible reaction.
•
At equilibrium, the:
•rate of the forward reaction is the same as the rate of the reverse reaction
•concentrations of the reactant and products remain constant
macroscopic properties are constant
how can graphs represent equilibrium
rates of reaction
concentrations of the substances over time
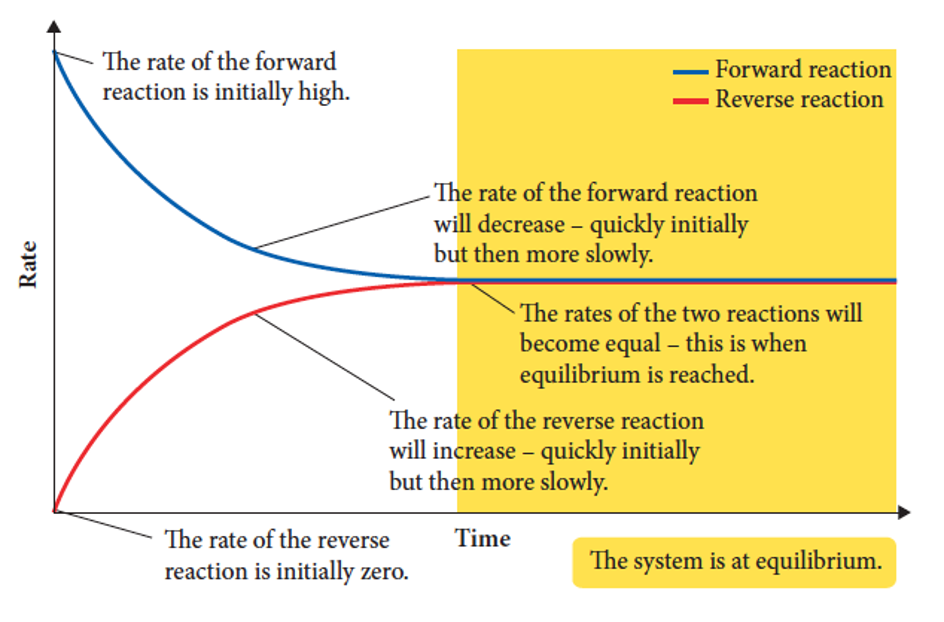
what is present in a reaction rate and time graph
1) The rate of forward reaction is initially high
2) The rate of reverse reaction is initially zero
3) rate of the forward reaction will decrease
4) rate of the reverse reaction will increase
5) The rates of the two reactions become equal and equilibrium is reached
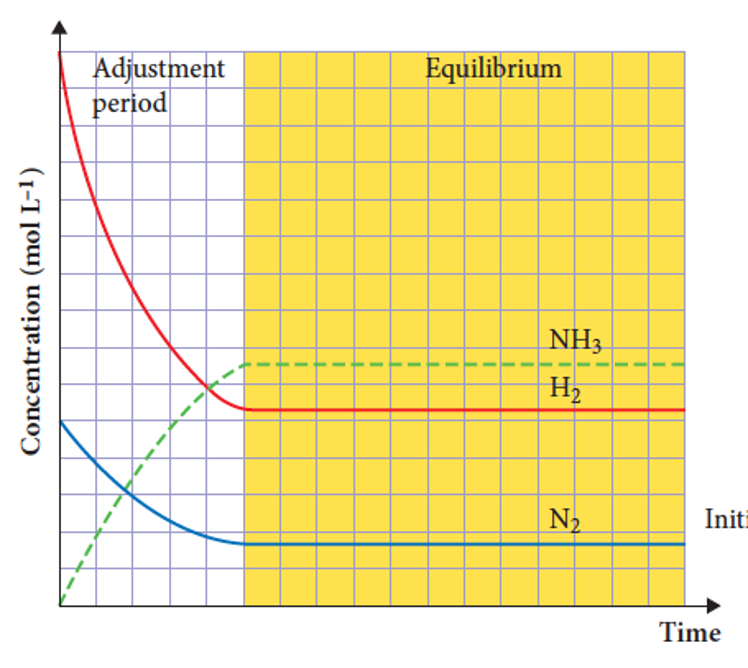
what is present in a concentration and time graph
1) The concentration of reactants is initially large
2) The concentration of the products is initially zero
3) concentration of reactants decreases, initially quickly, then more slowly.
4) The concentration of products increases, initially quickly, then more slowly.
5) all concentrations will plateau when the system reaches equilibrium
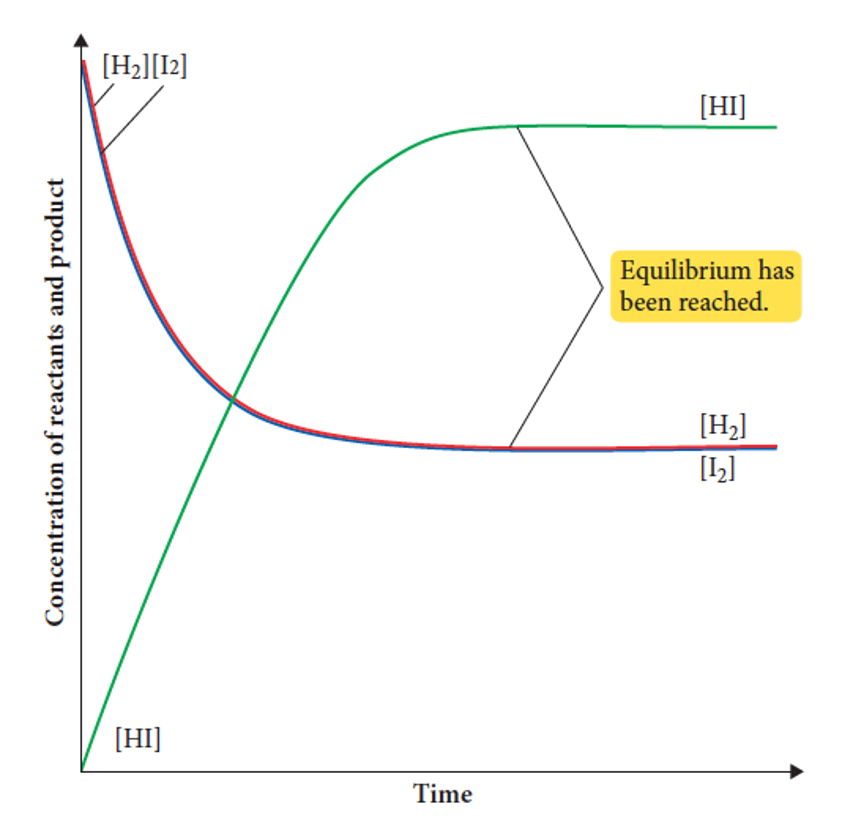
what is the difference between the two graphs in the number of lines
concentration vs time: any number depending on the number of reactants and products. Each may be represented on the graph by a separate line.
reaction rate: two lines - forward and reverse reaction
what is the difference between the two graphs in terms of the position of the line at equilbrium
Concentration vs time: all lines plateau as concentrations are constant. They do not need to be equal
reaction rate vs time: the two lines meet and plateau as the two rates are equal
what is the equilibrium constant ( also K)
the mathematical relationship between the concentrations of reactants and products. It is specific for a particular reaction at a particular temperature.
what do equilibrium constants predict
the relative concentrations of products and reactants and therefore the position of the equilibrium
how can the equilibrium constant be calculated
from the equilibrium expression

what is the K constant based on
the ratio of concentrations of reactants and products. the equilibrium constant is equal to K when the system is at equilibrium.
what substances are included and not included in the expression
Solutions and gases vary in concentration so are included in the expression.
Solids and liquids do not vary in concentration.
• They are NOT included when the system is heterogeneous (contains more than one phase).
• They ARE included when the system is homogeneous (all solid or all liquid).
what is the reaction quotient (Q)
the value that can used to determine whether the forward reaction or reverse reaction is favoured.
the equilibrium constant is used to determine Q when the system is not at equilibrium.
If Q = K
then the system is at dynamic equilibrium
If Q > K
then there are more products and less reactants than at equilibrium.
If Q < K
then there are less products and more reactants than at equilibrium.
what does a large equilibrium constant mean
If the K value is large:
• the reaction goes towards completion
• more products are present than reactants
• equilibrium lies to the right.
what if the k vaule is small
• the reaction only occurs to a small extent
• more reactants are present than products
• The equilibrium lies to the left.
what if the k value is close to 1
then there are significant concentrations of both products and reactants present.
what is yield
how much product can be produced
Yield is dependent on position of equilibrium.
Reactions with low K values have low concentration of products and hence low yield.
The rate of reaction indicates how quickly this yield will be achieved.
what happens when temperature, concentration or partial pressure of an equilibrium system is changed
The ratio of products and reactants and changes.
The system will favour either the forward or reverse reaction to return the system to equilibrium.
This often results in an overall increase in a particular species in the system.
how can collision theory be applied
When concentration of a reactant is increased (including pressure increases), collision theory states rate of reaction will increase due to increased collisions.
•This uses additional reactant, reducing its concentration, reducing rate of forward reaction.
•Additional product will form so rate of reverse reaction increases.
•Eventually rates of the two reactions equalise.
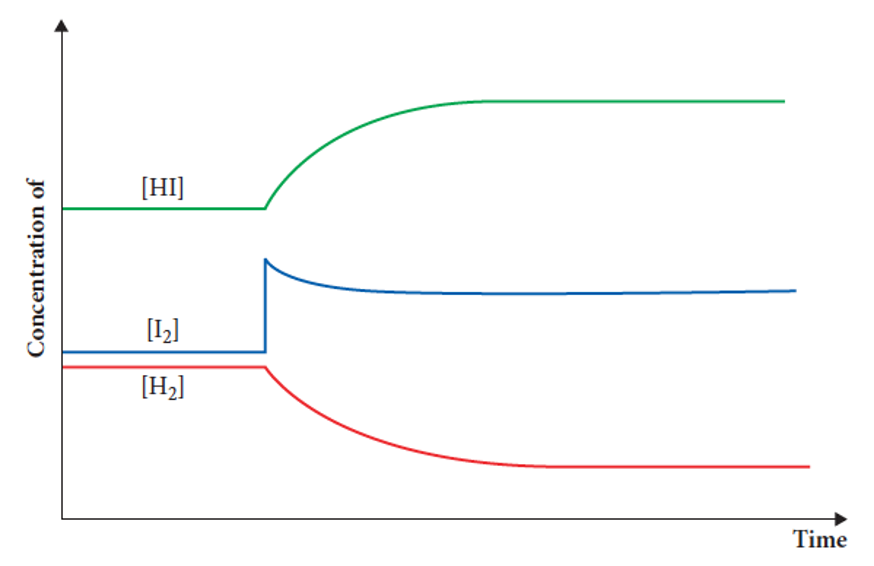
describe what happens here
• Iodine is added to the system, increasing the rate of the forward reaction.
• Concentration of iodine and hydrogen is reduced, while hydrogen iodide increases.
As more hydrogen iodide is produced, the rate of the reverse reaction increases.
• This pattern continues until the forward and reverse rates are equal and equilibrium is re-established (parallel lines on the graph).
what happens when a volume or pressure of a system is changed
the concentration of all gaseous species is also changed
The species that changes most has the greatest effect on equilibrium change.
The partial pressure of a gas and its concentration are related because they are both due to the number of particles in a given volume. A decrease in concentration leads to a decrease in partial pressure of that substance and rate of reaction.
in a endothermic reaction -
the products have more enthalpy than reactants, so energy is absorbed from the surroundings.
In an exothermic reaction -
reactants have more energy so energy is lost to the surroundings.
note that exothermic reactions have lower activation energy than exothermic.
when temperature is increased -
both forward and reverse reaction rates increase, but the endothermic rate will increase more.
A greater percentage of particles are able to react in the endothermic reaction due to the higher activation energy.
More endothermic products are produced, so the exothermic rate increases. Eventually equilibrium is re-established.
therefore, the equilibrium shifts to favour the endothermic reaction
what is le chatelier’s principle
if a system at equilibrium is subject to change in conditions, then the system will behave in a way to counteract that change.
what must you consider to predict changes in a system
1 What is the change?
2 What is the opposite of the change?
3 Which reaction is favoured – forward or reverse?
4 Does equilibrium shift left or right?
5 What happens to the concentrations of each substance?
if the concentration of a reactant is increased -
• the system responds to decrease it by favouring the forward reaction, using up the reactant
• equilibrium shifts to the right, increasing the concentration of the products and decreasing the concentration of reactants.
Note that the system does not fully counteract the change so the reactant concentration does not fully return to its initial levels
reactant increases in concentration or partial pressure or concentration
forward reaction is favoured
equilibrium shifts to the right
decrease in concentration or partial pressures of reactants
increase in concentration or partial pressure of products
reactant decreases in concentration or partial pressure or concentration
reverse reaction is favoured
equilibrium shifts to the left
increase in concentration or partial pressures of reactants
decrease in concentration or partial pressure of products
product increases in concentration or partial pressure or concentration
reverse reaction is favoured
equilibrium shifts to the left
increase in concentration or partial pressures of reactants
decrease in concentration or partial pressure of products
product decreases in concentration or partial pressure or concentration
forward reaction is favoured
equilibrium shifts to the right
decrease in concentration or partial pressures of reactants
increase in concentration or partial pressure of products
if the volume of a gaseous system changes
The concentration of all aqueous or gaseous substances changes
The system will react to reverse this change and restore initial concentrations
To predict what happens, we look at the number of gas molecules in reactants and products
if the volume of the system is decreased
then the pressure of the system increases.
the system will react to decrease the pressure by reduing the number of gas molecules.
the reaction that will be favoured is the one that has the smaller production of gaseous particles (look at the coefficient)
here the forward reaction is favoured and more ammonia is produced.
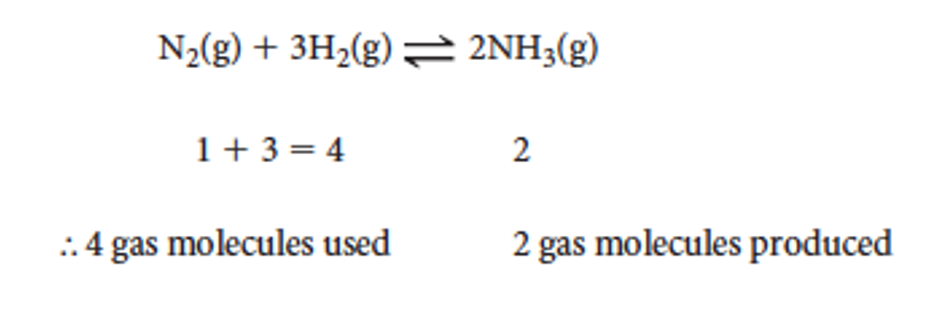
if a volume of a system is increased
increasing volume (decreasing pressure) shifts it towards the side with more moles of gas
if a temperature is increased
added energy is present, and to oppose this change the endothermic reaction is favoured to absorb this energy
if temperature is decreased
exothermic reaction is favoured to produce heat energy to oppose this change