specific heat capacity
1/7
Earn XP
Description and Tags
Different materials require a different amount of energy to change their temperature. The amount of energy required to raise 1kg of a material by 1°C is known as the specific heat capacity. In this practical we will experimentally identify the specific heat capacity of different materials by heating them and recording their change in temperature.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
aim
determine the specific heat capacity of a solid block by measuring the amount of energy required to change its temperature
independent variable
type of material
dependent variable
temperature rise
control variables
mass of material
surface area of material
equipment
power pack set to DC, 12V
metal blocks (e.g. aluminium, copper, steel)
immersion heater
ammeter
voltmeter
stop clock
insulating material
pipette with small amount of water
thermometer
how to know energy supplied (J) to immersion heater
P = IV for power
E = Pt for energy
setup
thermometer and immersion heater in block
insulator material around block
an ammeter connected in series with the immersion heater
a voltmeter connected in parallel with the immersion heater
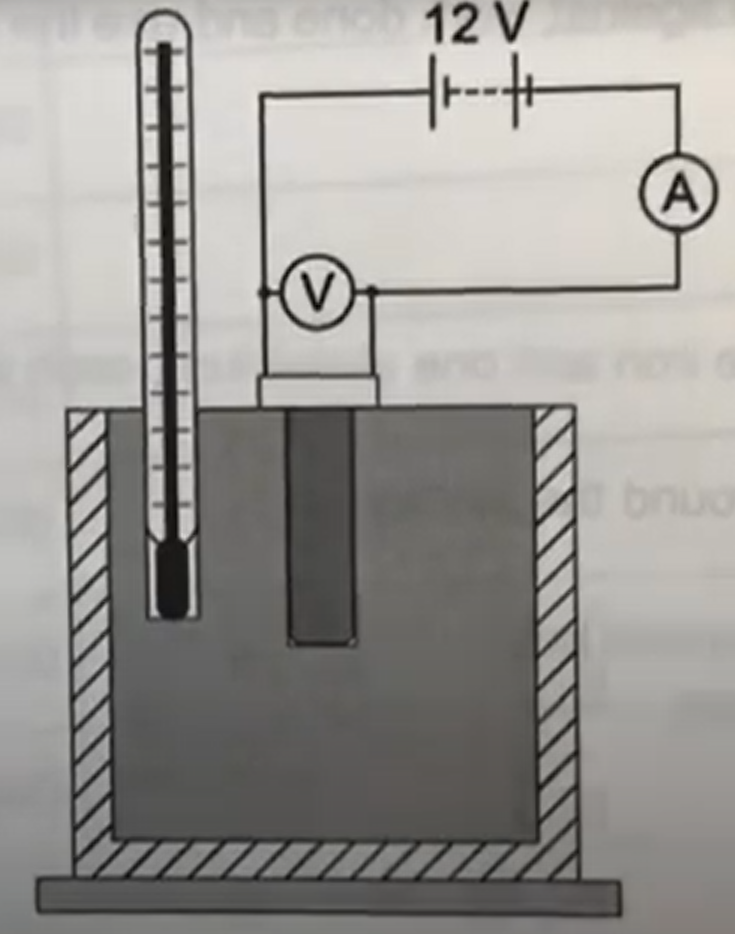
steps
add a few drops of water in the block where the thermometer goes
place the thermometer in the hole
record the initial temperature of the block
turn power pack on and start the stop clock
record the current and potential difference readings
when the stop clock reaches 1 minute, record the final temperature reading
repeat for 10 minutes