chem honors ch 1-2
1/41
Earn XP
Description and Tags
intro, matter, energy
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
matter
everything that has mass (all physical objects)
macroscopic
visible to the naked eye
microscopic
seen with a microscope
particulate
cannot be directly seen (the particles that make up matter)
macro, micro, and particulate behavior of matter
knowing how to control particles allows you to also control macro or micro behaviors
model
in chemistry:
representation of particulate-sized matter (atoms, molecules)
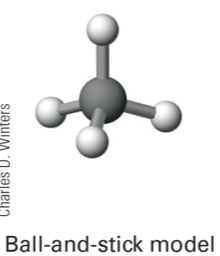
ball-and-stick model
shows atoms as balls and linking electrons as sticks
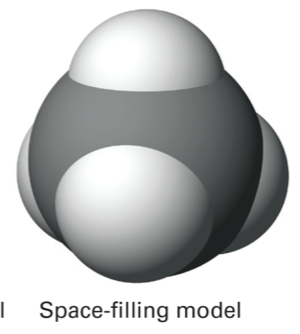
space-filling model
shows the outer boundaries of a particle (3D)
Kinetic Molecular Theory
all matters consists of (extremely tiny) particles that are in constant motion
*kinetic = motion, molecular = molecule
heat + states of matter
increases particle speed
molecules are _________ to one another
attracted
gas
fast-moving molecules, that can overcome attractive forces (don’t touch/far apart)
gas shape & volume
shape - same as closed container
volume - same as closed container
liquid
slower moving molecules, that touch one another but can still move freely amongst themselves
liquid shape and volume
shape - same as container (bottom bc of gravity)
volume - constant/fixed
solid
molecules stuck together in fixed positions, can only vibrate or shake
solid shape and volume
shape - constant/fixed
volume - constant/fixed
crystalline solid
molecules are fixed and arranged in a pattern (ex. table salt, snowflakes)
amorphous solid
molecules are fixed but with no pattern (ex. rubber, types of plastic/glass)
physical properties
characteristics observed and recorded without altering the substance’s identity (ex: color, density, hardness, boiling point)
physical change
change in the form of a substance without changing its chemical identity (ex. size, shape, phase/state change)
chemical change
when the chemical identity of a substance is destroyed, and a new substance forms (aka chemical reaction)
(ex. breaking the bonds in water to create hydrogen gas and oxygen gas)
chemical properties
all the possible chemical changes/reactions of a substance (how a substance reacts with other substances or energy)
(ex. water turns into H and O when exposed to an electric current)
chemical change indicator examples
change in color, release of heat or light (exothermic reaction), gas formation
pure substance
substance made up of a single chemical (one type of molecule)
own set of physical and chemical properties
identity doesn’t change from physical change
mixture
sample of matter made of two or more pure substances mixed together
properties of mixture depend on substances in it
homogenous
a mixture that is uniform in appearance and composition throughout (also called a solution)
*a pure substance is also homogenous
phase
the different separate, distinct layers forming a heterogeneous mixture (ex. oil and water layers)
heterogenous
a mixture with different phases visible to the naked eye
distillation
uses physical change (evaporation and condensation) to separate part of a mixture (typically water)
filtration
using the physical properties of a mixture to separate components (ex. separating different phases created by density)
element
cannot be decomposed or separated chemically into stable pure substances (or just different substances in general)
compound
can be decomposed by chemical change into other pure substances (ex. H20)
chemical formula
symbols representing the atoms that make up a compound (ex: Na, H20)
atom
smallest particle of an element
molecule
smallest unit particle of a pure substance (can be an element or compound)
Law of Constant Composition
“Any compound is always made up of elements in the same proportion by mass”
the mass of a compound equals the mass of its elements in their set proportions
(basically saying mass a + b = c)
static electricity (electrostatic force)
forces of attraction or repulsion between electrically charged objects
Energy
ability to do work or transfer heat
exothermic vs endothermic
exothermic (out) - chem change that transfers energy into surroundings
endothermic (in) - chem change that removes energy from surroundings
kinetic vs potential energy
kinetic - energy due to the motion of an object
potential - energy possible/stored through the particle’s arrangement or position (ex. sucrose for plants or gravitational potential energy)
Laws of Conservation (3 of them)
Mass and Energy - total quantity of mass and energy in universe is fixed (does not change)
Mass - total mass of reactants in a chemical change = total mass of products
Energy - quantity of energy within an isolated system does not change