Water Supply Week 11
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
total solids
residue on evaporation at 103C
measuring solids
place known value of sample (water + solids) in evaporating dish
allow water to evaporate (provide heat)
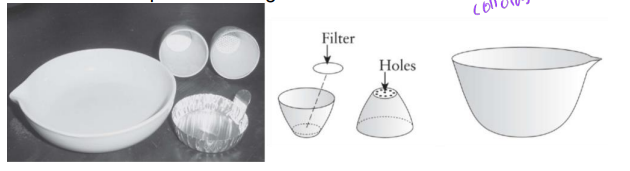
gooch crucible (with bottom holes) to separate suspended solids from dissolved solids
place glass fiber filter on holes in crucible
sample drawn through crucible with aid of vacuum
suspended material is retained on filter
dissolved fraction passed through filter
classification of solids
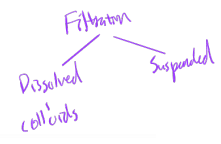
total solids = dissolved solids + suspended solids
dissolved solids: salts, colloidal matter (i.e., not filterable)
suspended solids: sand particles, silt (filterable)
TS = DS + SS
total solids = volatilized solids + fixed solids
volatile solids = solids that volatilized at elevated T
fixed solids = solids that survived high-T treatment
TS = VS + FS
Fixed solids (FS)
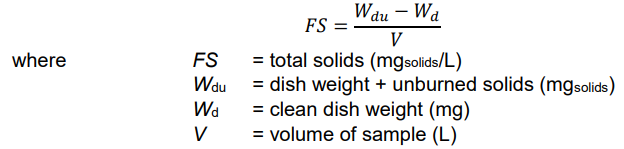
volatile solids (VS)
VS = TS - FS
volatile suspended solids (VSS)
determined by placing gooch crucible (with filtered content) in hot oven (600C) to burn organic fraction and weighed again
loss in weight = VSS
Ex. Water sample has following laboratory measurements
• weight of crucible = 48.6212 g
• 100 ml of sample was placed on the crucible; after evaporation at 103oC, weight of crucible + content = 48.6432 g
• crucible (+ content) was placed in a 600oC furnace for 24 hours and subsequently cooled in a desiccator; weight of crucible + residue = 48.6300 g
Determine Total Solids, Volatile Solids, and Fixed Solids from the above measurements.
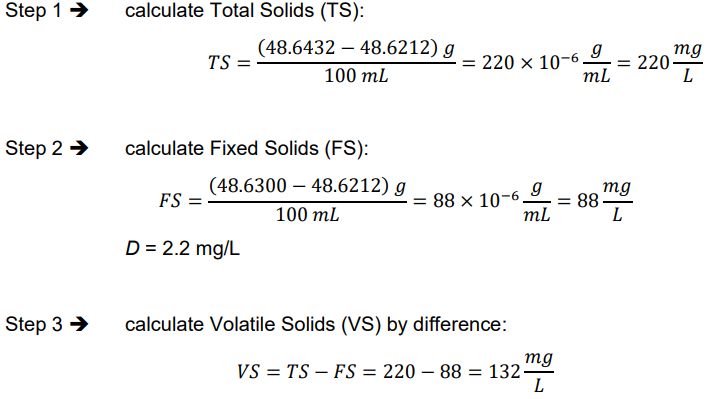
pathogens
microorganisms that may potentially cause diseases/illnesses in humans
infectious diseases such as typhoid and cholera can be transmitted by water
common waterborne microbial pathogens in table below
what do we do with pathogens? how do we measure presence?
common waterborne pathogens
indicator organism: E. coli
measure bacteriological quality using indicator organism – species who presence indicates the likely presence of pathogens
common indicator – Escherichia coli (E. coli), member of the coliform bacteria group; E. coli a.k.a. fecal coliforms that are found in digestive tracts of warm-blooded animals
presence of coliforms indicates rather than proves, the likely presence of pathogens in a given sample; thus not definitive or suggestive
E. coli is present in other animals, so seeing E. coli in water means animals deification likely contaminated the water, NOT because it always harms humans
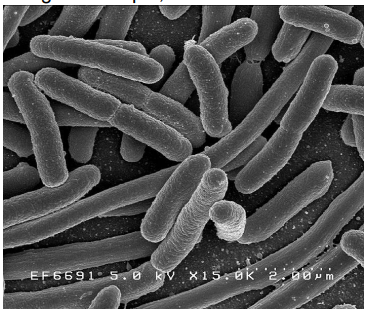
measurement of fecal coliforms: (1) membrane filter technique (MF)
water sample filtered through sterile micropore filter
coliforms present are captured on filter
the filter placed in Petri dish with a culture medium that promote fecal coliforms growth but suppress other microbes
incubate for 24-h at 35oC
count number of shiny metallic red dots (each representing one fecal coliform colonies)
concentration of coliforms [coliforms/100 ml sample] reflects bacteriological quality
measurement of fecal coliforms: (2) most probable number (MPN)
coliforms metabolize lactose to produce gas and make lactose solution cloudy
gas produced and cloudy broth after incubation indicates presence of coliform
MPN used on very turbid, brackish, muddy water sample
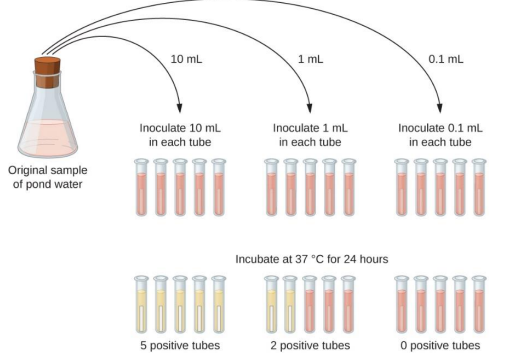
MPN: record the number of positive responses with different amounts of water sample (potentially with microbes) and determine coliform concentrations using a standard scorecard
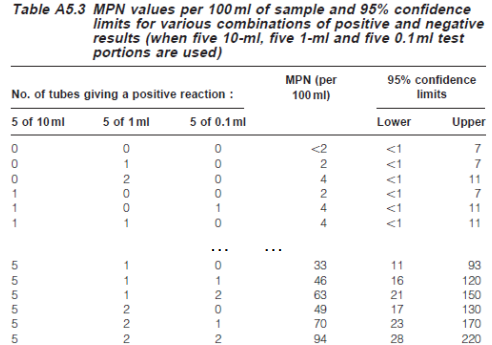
For the test results above (i.e., 5 +ve with 10 ml, 2 +ve with 1 ml, 0 +ve with 0.1 ml), the scorecard gives 49 coliforms / 100 mL

other water quality parameters / concerns
toxic to human and other aquatic organisms often at low/trace levels
exert a wide suite of toxicological effects
acute and chronic effects ➔ toxicology / ecotoxicology
can accumulate and transfer along the food web
addressing these contaminants effectively and comprehensively is challenging
heavy metals / trace elements
commonly monitored and regulated species:
arsenic (Ar), lead (Pb), selenium (Se), chromium (Cr), cadmium (Cd), cobalt (Co), nickel (Ni), antimony (Sb), mercury (Hg)
organic pollutants
- volatile organic compounds (VOCs)
- persistent organic pollutants (POPs)
limitations of standard water quality parameters
overlook new pollutants (micropollutants and emerging contaminants
e.g., perfluorinated chemicals such as PFOAs, PFOSs pharmaceuticals and personal care products pesticides (or “plant protection products” – PPP) (and many more….)
coagulation purpose
to remove colloids (<1 um) present in natural waters
not easily removed by sedimentation and filtration
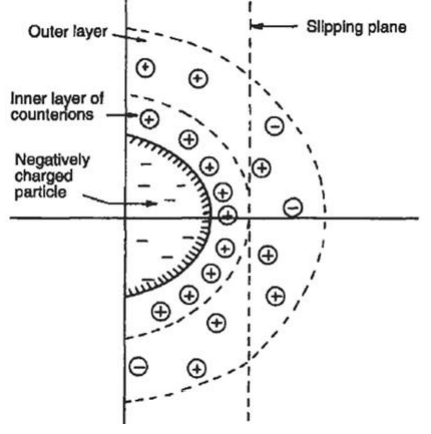
stability of colloids
colloids are so small (dia. ~< 1 m) that they are very slow to settle out
colloids in natural waters carry negative surface charge
negative surface charge prevents formation of stable aggregates
how can we destabilize the colloids?
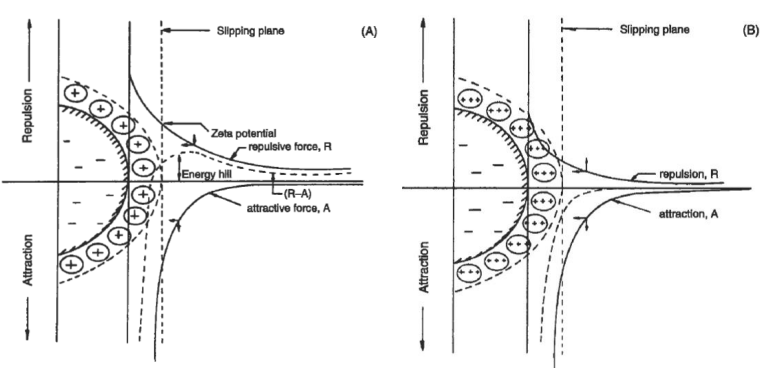
colloids surface charge
introduction of multi-valent ions can neutralize the negative surface charge of colloids, bringing down the repulsive forces
coagulation ➔ adding trivalent cations to reduce the energy barrier
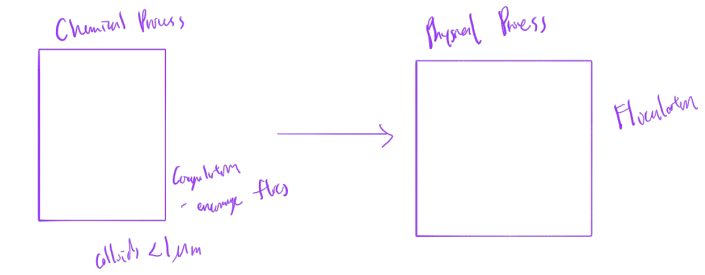
coagulation mechanisms
a. charge neutralization
b. interparticle bridging
c. enmeshment in a precipitate (“sweep floc”)
charge neturalization
destabilized particulates by adsorption of oppositely charged ions/polymers
particulates in natural water are negatively charged (e.g., clays, humic acids, bacteria, etc.); pH ~ 6 to 8
metal salts, and cationic organic polymers can encourage particulate aggregation through charge neutralization
optimum coagulant dose occurs when the particle surface is only partially covered (< 50%)
optimum dose generally increases with particulate surface are
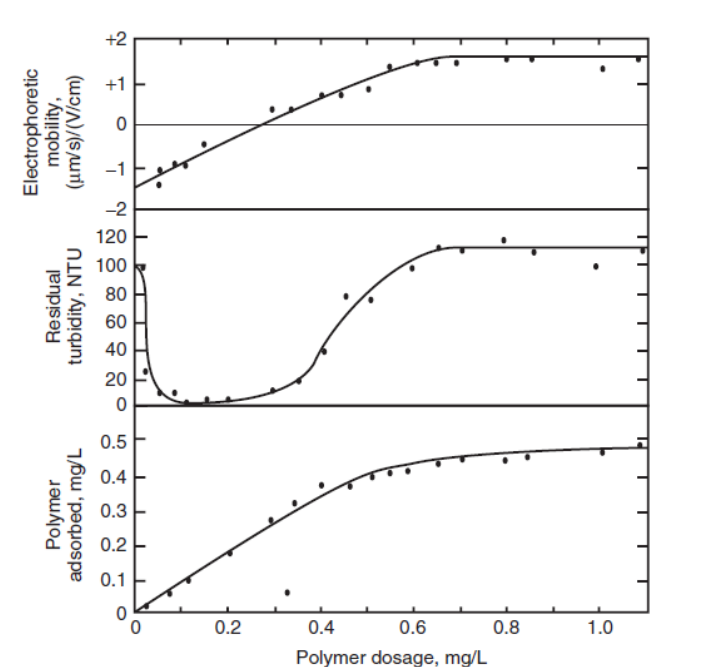
polymeric coagulant and dose
particle will coagulate when optimal amount of polymer has adsorbed to the particulate and neutralize its surface charge
too much polymer ➔ particles remain +ve charged ➔ no aggregation
low dose polymer ➔ sub-optimal coagulation
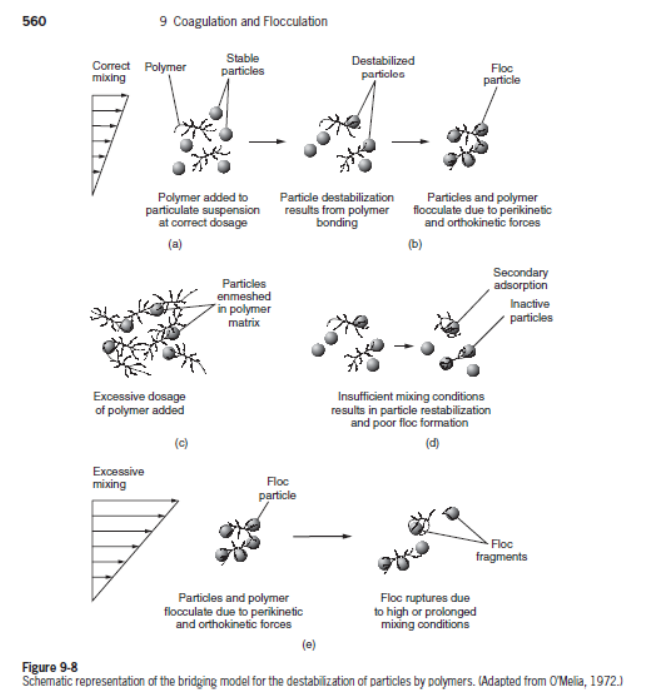
interparticle bridging
large polymer molecules link up different particulates and form a large aggregate
polymeric bridging is an adsorption phenomenon ➔ optimal bridging dose proportional to particulates present
bridging occurs with nonionic polymers and high-molecular-weight (MW 105 – 107 g/mol), low-surface-charge polymers
bridging efficiency = f(polymer size/length, charge, and charge density)
polymer selection based on empirical testing
dose effect on polymer bridging:
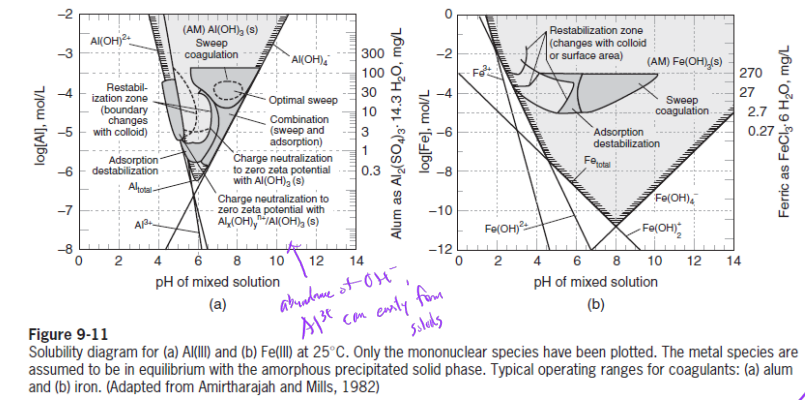
Precipitation and Enmeshment (or “Sweep Floc”)
entrapment of particulate during aluminum (Al) and iron (Fe) precipitates formation (which occur at high metal dosages)
steps:
1. hydrolysis and polymerization of metal ions
2. adsorption of hydrolysis products at interface
3. charge neutralization
4. at high metal ion dosages, nucleation of the precipitate occurs at the particulate surface, leading to growth of amorphous precipitate with the entrapment of particles in this amorphous structure
entrapment mechanism dominates in pH 6 ~ 8 and applications of Al and Fe salts
sweep floc mechanism does not depend on type of particles (i.e., particles’ chemical makeup)
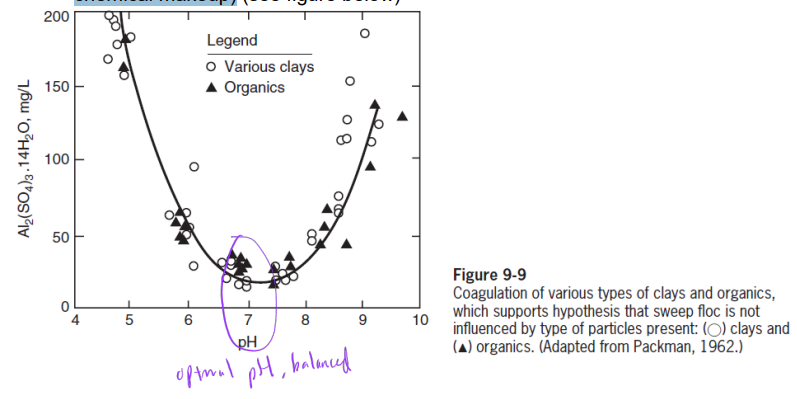
optimal pH range for sweep floc mechanism also consistent with solubility of Al3+ and Fe3+: