Topic 15: Chromium lore
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Show equations displaying the amphoteric nature of chromium hydroxide
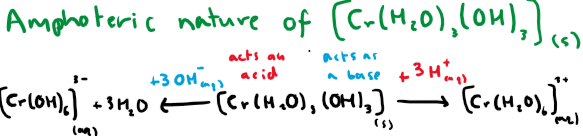
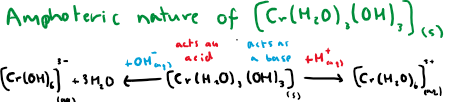
What type of reactions are these?
NOT ligand exchange reactions, these are acid-base reactions. Products are chemically changed through addition or removal of H+ ions.
What are the steps for making chromium ethanoate?
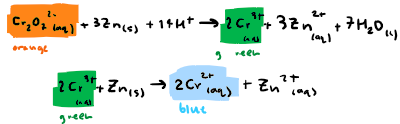
Reduce acidified sodium dichromate (VI) using zinc
React sodium ethanoate with Cr2+
What is the equation for this step: Reduce acidified sodium dichromate (VI) using zinc? State any colours
What is the equation for this step: React sodium ethanoate with Cr2+? State any colours
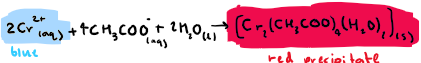
What are the conditions needed for making chromium ethanoate and why?
Experiment must be done in inert conditions,
What type of atmosphere is usually used for this experiment?
Nitrogen atmosphere