1.4.2 Enzymes
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
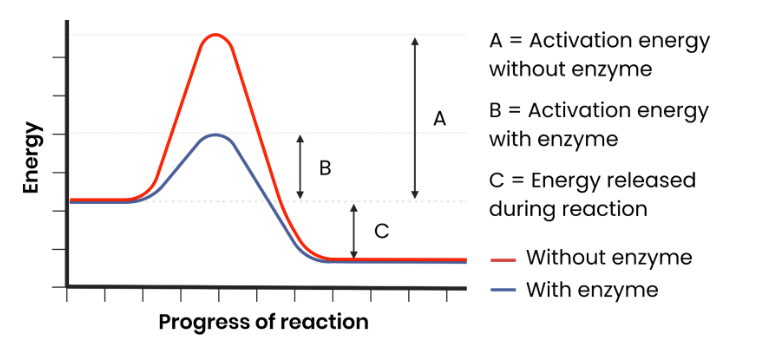
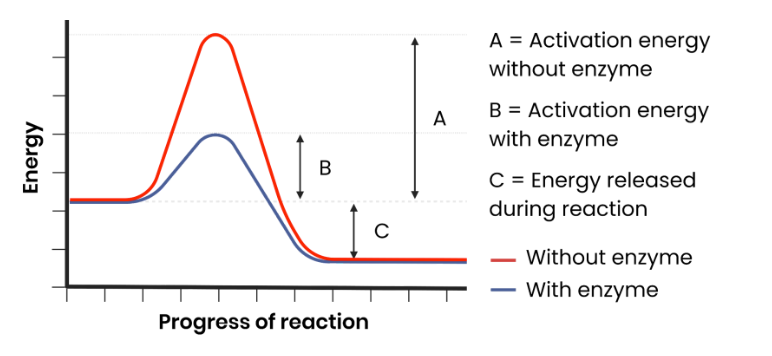
How do enzymes act as biological catalysts?
Each enzyme lowers activation energy of reaction it catalyses, to speed up rate of reaction.
Describe the induced-fit model of enzyme action
Substrate binds to the (not completely complementary) active site of enzyme, causing active site to change shape slightly so it is complementary to its substrate. Enzyme-substrate complex forms, causing bonds in substrate to bend/ distort, lowering activation energy.
Explain the specificity of enzymes
Their specific tertiary structure determines shape of active site which depends on sequence of amino acids in the primary structure. Active site is complementary to a specific substrate. Only this substrate can bind to active site, inducing fit and forming an enzyme-substrate complex.
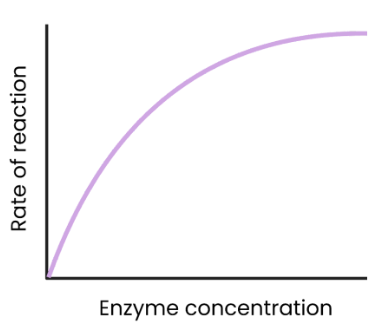
What is the effect of enzyme concentration on the rate of enzyme-controlled reactions?
As enzyme concentration increases, rate of reaction increases.
more enzymes so more available active sites, so more enzyme-substrate complexes form.
At a certain point, rate of reaction stops increasing/ levels off as the substrate concentration becomes the limiting factor (all substrates in use)
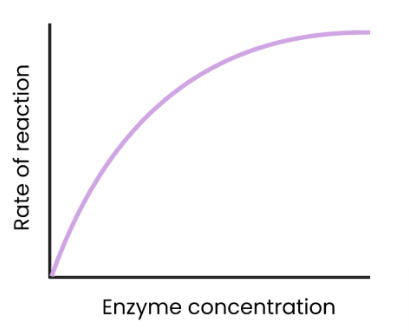
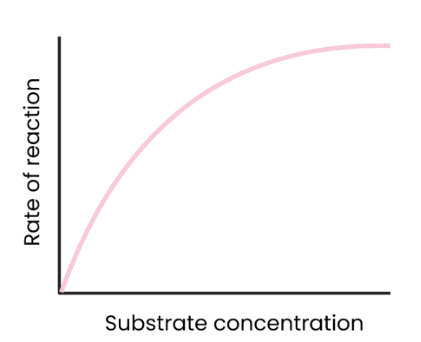
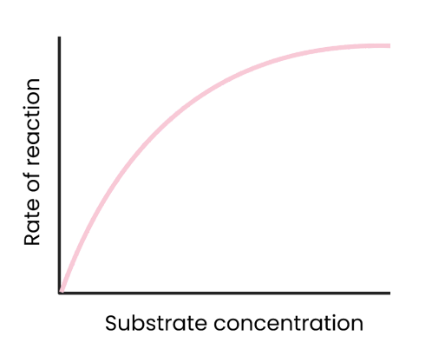
What is the effect of substrate concentration on the rate of enzyme-controlled reactions?
As substrate concentration increases, rate of reaction increases.
More enzyme-substrate complexes form.
At a certain point, rate of reaction stops increasing/ levels off as enzyme concentration becomes the limiting factor. (all active sites are occupied)
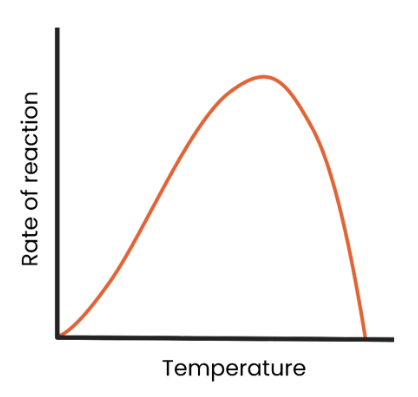
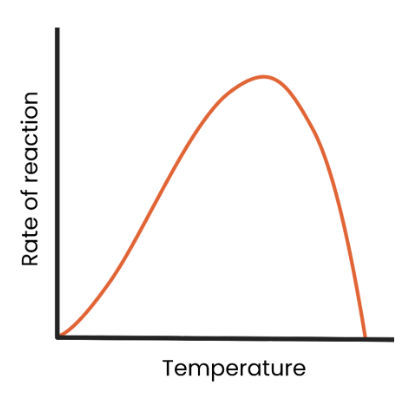
What is the effect of temperature on the rate of enzyme-controlled reactions?
as temperature increases to optimum, rate of reaction increases as kinetic energy increases, so more enzyme-substrate complexes form.
as temperature exceeds optimum, rate of reaction decreases as the enzyme denatures (the tertiary structure and active site change shape) as hydrogen/ ionic bonds break.
The active site is no longer complementary so fewer enzyme-substrate complexes form.
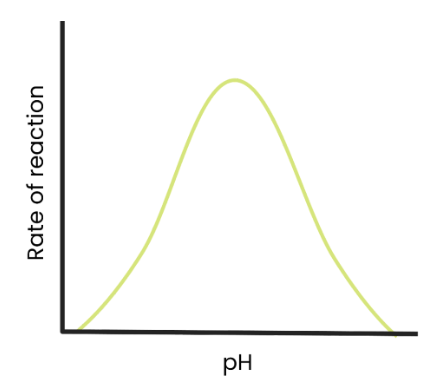
What is the effect of pH on the rate of enzyme-controlled reactions?
As pH increases above an optimum, the rate of reaction decreases as the enzymes denature (the tertiary structure and active site change shape) as hydrogen/ ionic bonds break.
The active site is no longer complementary so fewer enzyme-substrate complexes form.
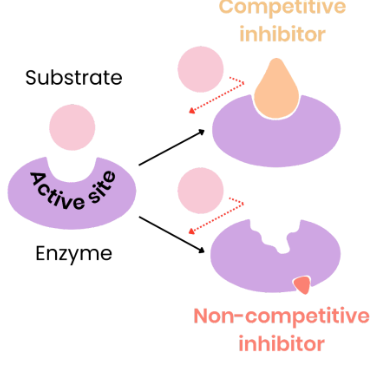
How does a competitive inhibitor effect enzyme-controlled reactions?
As concentration of competitive inhibitor increases, rate of reaction decreases.
- similar shape to substrate, competes for active site to block it so substrates cant bind. Fewer enzyme substrate complexes form.
Increasing substrate concentration reduces effect of inhibitors - not permanent.
How does a non-competitive inhibitor effect enzyme-controlled reactions?
As concentration of non-competitive inhibitor increases, rate of reaction decreases.
- binds to allosteric site and changes enzyme tertiary structure/ active site shape so its no longer complementary to substrate. Substrates can not bind so fewer enzyme-substrate complexes form.
Increasing substrate concentration has no effect on rate of reaction as change to active site is permanent.