molecular geometry
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Angle between 2 bonds is called the…
bond angle
bond angle for a linear molecule is…
180o
when we predict the shape of a molecule we assume that the bonding pairs…
repel as far as possible
trigonal planar bond angle?
120o
if a molecule has 3 electron pairs, we say that its shape is…
trigonal planar
if a molecule has 4 bonding pairs and 0 lone pairs its shape is…
tetrahedral
tetrahedral bond angle…
109.5o
as the number of lone pairs in a molecule increase the…
bond angle decreases
an electron pair exists in a——— cloud
charge
lone pairs repel more than bonding pairs so if theres a lone pair the distance between two bonding pairs…
decreases
a lone pair has…
a bigger charge cloud than a bonding pair therefore repels more than bonding pairs
we would expect the repusion between a lone pair and a bonding pair to be…
1) less than the repulsion between 2 lone pairs
2)more than the repulsion between 2 bonding pairs
The order of the electron pair repulsion is:
lone pair-lone pair >lone pair-bonding pair> bonding pair-bonding pair
why is the bond angle smaller in ammonia than in methane?
CH4 has 4 bonding pairs of electrons
NH3 has 3 bonding pairs and 1 lone pair
lone pairs take up more space and repel more strongly than bonding pairs
so bonding pairs in NH3 are pushed closer together and bond angle is closer together
If a molecule has 2 bonding pairs and 1 lone pair its shape is…
bent
bond angle for bent shape is…
118o
if a molecule has 3 bonding pairs and 1 lone pair we say that its shape is…
trigonal pyrimidal
bond angle of trigonal pyrimidal shape…
107o
If a molecule has 5 electron pairs and 0 lone pairs, we say that its shape is…
trigonal bipyrimidal
The bond angles for a trigonal bipyramidal molecule are...
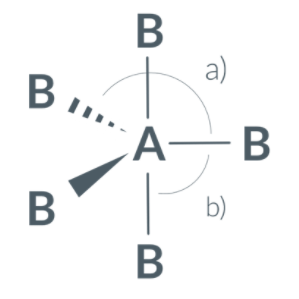
a)120o
b)90o
The shape the electron pairs form is…
trigonal bipyramidal
If a molecule has 4 bonding pairs and 1 lone pair, we say that its shape is...
sawhorse.
seesaw.
trigonal bipyramidal.
the bond angles for a seesaw are..
102o
87o
If a molecule has 3 bonding pairs and 2 lone pairs, we say that its shape is...
And the bond angles are...
T-shaped
84o and 90o
If a molecule has 2 bonding pairs and 3 lone pairs, we say that its shape is...
And the bond angle is...
linear
180°
Name this molecular shape and state the sizes of its bond angles.

Name:
seesaw
Angle X:
102°
Angle Y:
87°
ICl3 has 3 bonding pairs and 2 lone pairs. Which of the following statements is true?
The arrangement of the electron pairs is trigonal bipyramidal but the atoms are t-shaped
Which of the following electron pair combinations give molecules a linear shape??
2 bonding pairs and 0 lone pairs
2 bonding pairs and 3 lone pairs
explain why the fluorine atoms in XeF2 occupy a linear geometry.
electron pairs occupy a trigonal bipyramidal arrangement.
There are 2 bonding pairs and 3 lone pairs.
Lone pairs of electrons take up more space and repel more strongly than bonding pairs.
If fluorine atoms occupy the positions above and below the plane, it ensures that lone pairs are maximally separated from one another (120°).
What are the bond angles in this molecule??
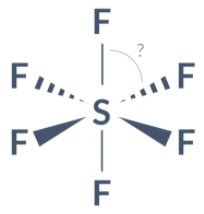
90o
What are the bond angles in this molecule??

90o
If a molecule has 6 electron pairs, we say that its shape is...
And the bond angles are...
octahedral.
90°
If a molecule has 4 bonding pairs and 2 lone pairs, we say that its shape is...
And the bond angles are...
square planar.
90°
Which of the following molecular shapes contain 90° bond angles??
Trigonal bipyramidal
Square planar
Octahedral
bond angle of 2 lone pairs and 2 bonding pairs…
104.5o
planar
In planar molecules, all atoms lie in a single plane
Explain why AsH3 has a trigonal pyramidal shape.
There are 4 electron pairs around the arsenic atom.
The 4 electron pairs repel each other to form a tetrahedral arrangement, where they are maximally separated from one another.
Of the 4 electron pairs, 3 are bonding pairs and 1 is a lone pair.
Lone pairs repel more strongly than bonding pairs.
So, the molecule has a trigonal pyramidal shape with a 107° bond angle.
What is the bond angle in CO2??

180o
When we predict the shape of a molecule with a double bond, we assume that...(2)
electron pairs in a double bond don’t repel each other
the double bond repels a similar amount to a single bond
When two atoms share a single pair of electrons, the electrons exist in a(n)...
sigma bond
When two atoms share a second pair of electrons, the electrons exist in a(n)...
pi bond
Using the dot and cross diagram, predict the shape of HCN.
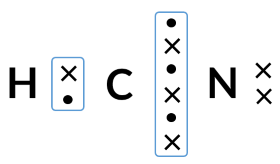
shape:linear
bond angle:180o
By drawing a dot and cross diagram, state the number of electron pairs around the central atom in ClF2+…
Both fluorine and chlorine have 7 electrons in their valence shell.
The ion has a positive charge, so we remove an electron from the least electronegative atom (chlorine).
