AP Biology - UNIT 1 ASSESSMENT: THE CHEMISTRY OF LIFE
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
77 Terms
Explain why shape is important for the following: Enzymes
Enzymes: Specific proteins that interact with a specific substrate. There are also competitive inhibitors.
Explain why shape is important for the following: Receptors
Receptors: Specific proteins usually embedded in the cell membrane by specific ligands. Hormones include testosterone, insulin, and estrogen. Ions are Na+ and K+. Medications include painkillers like opiates. Neurotransmitters include dopamine and serotonin.
Explain why shape is important for the following: Transport Proteins
Transport Proteins: Hemoglobin must have a biconcave shape to carry oxygen in red blood cells. Sickle cell disease is when the red blood cell is not the correct shape and barely functions.
Explain why shape is important for the following: Antibodies
Antibodies: Specific proteins made by white blood cells (B-cells) to interact and kill specific antigen (anything that causes an immune response; virus and bacteria).
Explain why shape is important for the following: Water
Water: It is bent.
Explain why shape is important for the following: DNA
DNA: DNA is a double helix which allows for DNA replication and transcription. When it is zipped closed, the genes are protected. Adenine has two hydrogen bonds with thymine, while guanine has three hydrogen bonds with cytosine.
Explain why shape is important for the following: Saturated vs. Unsaturated Fat
Saturated vs. Unsaturated Fat: They differ in which is filled with hydrogen or not. It determines whether it is liquid or solid at room temperature.
Explain why shape is important for the following: Plant Starch/Amylose
Plant Starch/Amylose: Can be broken down for energy because of its structure. However, cellulose and fiber can’t be broken down into energy because of their structure.
What is an element and compound?
An element is a substance that cannot be broken down into other substances by chemical reactions. A compound is a substance consisting of two or more elements in a fixed ratio. A compound is different from its elements.
What are the four main elements of life? What about the other 4%? Name some trace elements?
The four main elements are carbon, hydrogen, oxygen, and nitrogen. The other 4% are potassium, phosphorus, sulfur, and calcium. Trace elements include iodine (makes thyroxine and activates all body cells and controls metabolism), iron (found in hemoglobin and allows O2 to bond), and fluorine (absorbed in gums for strong teeth).
What is atomic number vs atomic mass?
Atomic number is the number of protons in its nucleus. Atomic mass is like the mass number which is the sum of protons and neutrons.
What is an isotope?
Isotopes are two forms of an element that differ in the number of neutrons.
What are radioactive isotopes used for?
Radioactive isotopes are used for dating fossils, tracing atoms through metabolic processes, and diagnosing medical disorders (PET scans find hotspots that are cancerous).
As an electron goes to a higher energy level, what happens to potential energy? What energy levels do electrons prefer to be in?
As an electron goes to a higher energy level, potential energy increases. Electrons prefer to be in the lowest energy level.
What are valence electrons?
Valence electrons are in the outermost shell. They mostly determine the chemical behavior of an atom.
What are covalent bonds? Electronegativity?
A covalent bond is the sharing of a pair of valence electrons by two atoms. A molecule is two or more atoms held together by covalent bonds. There can be single, double, or triple bonds. Electronegativity is an atom’s attraction to the electrons in a covalent bond. Oxygen, nitrogen, and sulfur are very electronegative. When the sharing of electrons are uneven it is polar, when they are even it is nonpolar.
What are ionic bonds?
Ionic bonds are when electrons are transferred. A cation is a positively charged ion. An anion is negatively charged. An ionic bond is an attraction between an anion and cation. They dissociate in water.
What is a hydrogen bond?
A hydrogen bond forms when a hydrogen atom covalently bonded to an electronegative atom (usually oxygen or nitrogen) is also attracted to another electronegative atom.
What is Van der Waals?
Van der Waals interactions are attractions between molecules that are close together as a result of these charges. Together they can get a bit strong, like the interactions between a gecko’s toe hairs and a well surface.
What is important about molecular shape? How does this relate to endorphins?
Molecular shape is very important to its function. In a covalent bond, the s and p orbitals may hybridize, creating specific molecular shapes. This relates to endorphins (which relieves pain temporarily), as morphine has a similar shape to it, so it can have the same effect. Unfortunately, it is super easy to get addicted to.
What is chemical equilibrium?
Chemical equilibrium is reached when the forwards and reverse reaction rates are equal.
What are acids and bases?
Acids are compounds that release hydrogen ions in water. Examples include vinegar and citrus fruits. Bases or alkaline are compounds that accept hydrogen ions and take them out of a solution (or they release hydroxide ions in water). Examples include baking soda, egg whites, and soap.
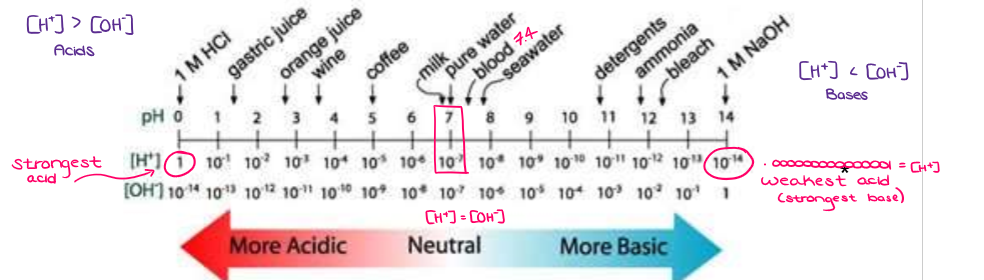
What is the pH scale?
The pH scale indicates the relative concentration of hydrogen ions. As hydrogen ion concentration increases, pH decreases. A pH of less than 7 is an acid, a pH of 7 is neutral, and a pH of more than 7 is a base.
What is denaturing?
Denaturing is when a protein loses its shape and function.
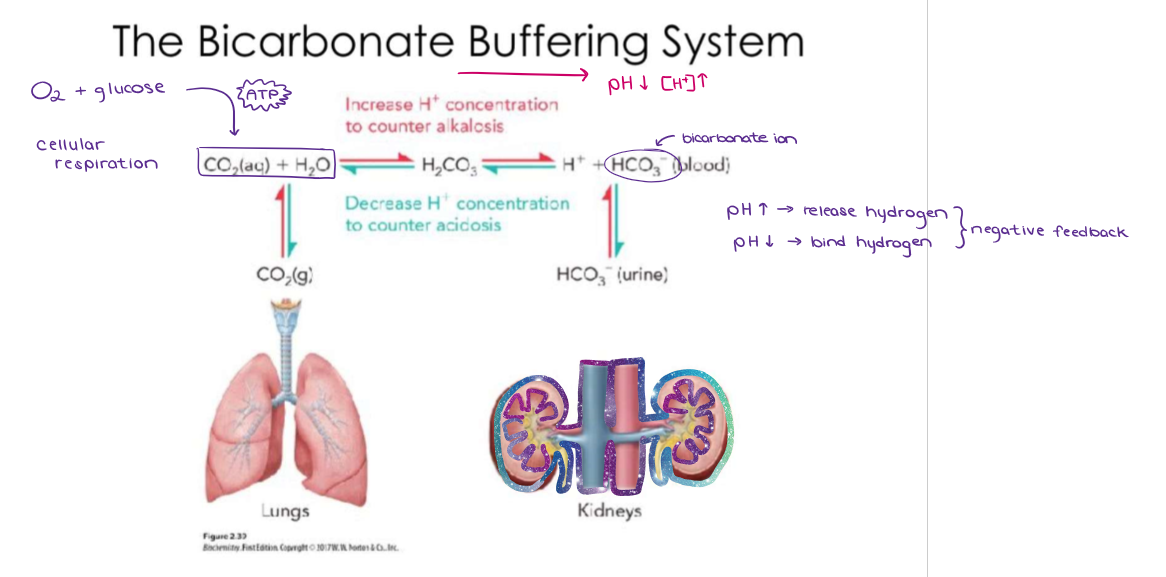
What are buffers? How is this seen in the carbonic acid - bicarbonate buffer?
Buffers are substances that prevent large fluctuations in pH. They can release hydrogen ions when the environment is too basic, or they can act as a base by accepting hydrogen ions when it is too acidic. The carbonic acid and bicarbonate buffer helps maintain the pH in blood sugar (at 7.4). Carbonic acid can release hydrogen ions to lower pH (more acidic). Bicarbonate can bind hydrogen ions to increase pH (more basic).
What is water? Its formula and structural formula?
Water is the most important inorganic molecule, it is ⅔ of our body, and most biological processes and chemical reactions can only occur there. It is H2O and has a bent shape. The oxygen is partially negative and the hydrogen ions are partially positive. It can form three hydrogen bonds as a liquid and four as a solid.
What happens to energy when hydrogen bonds are broken and formed?
When hydrogen bonds are broken energy/heat is absorbed. When they are formed, heat/energy is released.
What is a solution? Solvent? Solute?
A solution is a homogeneous mixture that consists of two parts. A solvent is the substance that dissolves another, while the solute is the substance being dissolved.
Why is water less dense as a solid?
Water is less dense as a solid because when water freezes, the hydrogen bonds stabilize and push the water molecules further apart. As the volume increases, the density decreases, causing ice to float.
What is cohesion and adhesion?
Cohesion is when water bonds to itself and adhesion is when water bonds with other molecules.
What are the emergent properties for cohesion? (4 of them).
The first emergent property for cohesion is a high surface tension which is when molecules on the surface of water form a tight network making it difficult to penetrate the surface. This allows cells to maintain their shape and some animals to travel on the surface of the water. The high boiling point is due to high energy being needed to break the hydrogen bonds before water evaporates. This allows bodies of water to not evaporate and makes the cooling effect of sweat possible. Water has a high heat capacity, as a lot of energy needs to be absorbed before the temperature rises. This helps regulate temperature in organisms and moderate temperature in coastal regions. Water is less dense as a solid, which allows life to survive during the winter. The ice insulates what is below it.
What are the emergent properties for adhesion? (2 of them).
The first emergent property for adhesion is capillary action (which also uses cohesion). It is the movement of a liquid through a narrow tube against gravity. This allows plants to distribute water through the xylem and for humans it is through the capillaries. Water is also a versatile solvent as charged regions on water attract ions and charged areas of polar compounds. It dissolves most substances, which allows chemical reactions to occur in our cells.
What is organic chemistry?
Organic chemistry is the study of compounds that contain carbon.
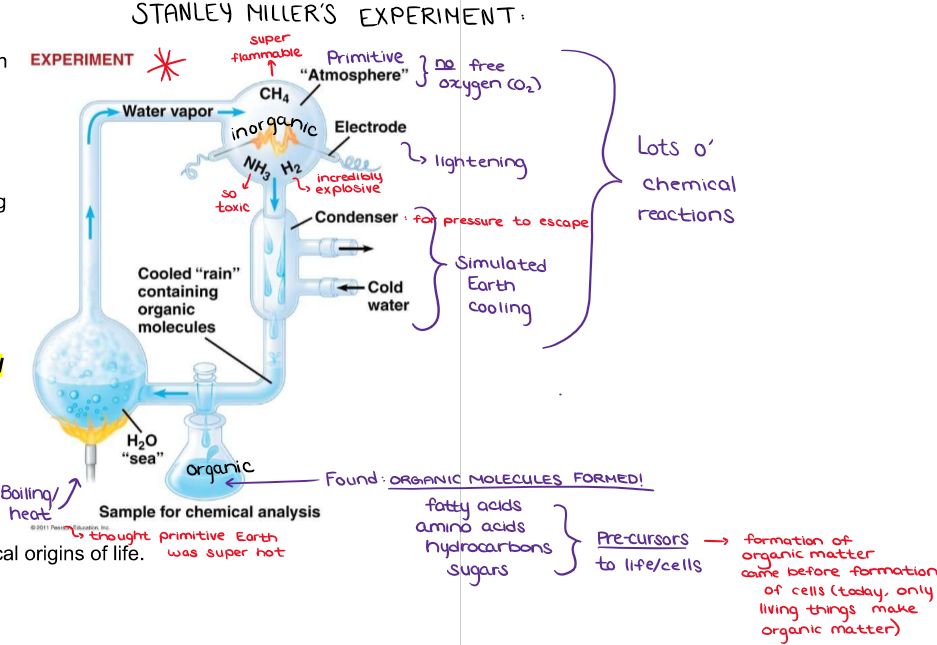
What is Miller’s experiment?
Designed to test the idea that Earth’s early atmosphere could have given rise to life’s building blocks under the right conditions
Miller simulated early Earth by combining water (to represent oceans), methane, ammonia, and hydrogen (to represent atmospheric gases) inside a closed apparatus (there was no free oxygen on early Earth)
Electrical sparks were passed through the gases to mimic lightning, a possible source of energy on young Earth
Miller found that several organic molecules had formed, including fatty acids, amino acids, hydrocarbons, and sugars, after running the experiment for a week
Showed that organic molecules necessary for life could arise spontaneously from inorganic components (so the formation of organic matter came before the formation of cells) under conditions thought to resemble early Earth
Today, only living things can make organic matter!
What are organic vs inorganic compounds?
Organic compounds contain hydrogen and carbon, they are large and complex, and they make up and are made by living things. It includes carbohydrates, proteins, nucleic acids, and lipids. Inorganic compounds do not contain carbon and hydrogen together, and they are not as large and complex.
What is special about carbon?
Carbon is special because it has four valence electrons and four covalent bonds. It can also bond to itself allowing there to be large organic molecules.
What is a hydrocarbon?
Hydrocarbons are composed of only carbon and hydrogen. They contain a lot of energy, as they have tons of bonds. They are always nonpolar and hydrophobic.
What are structural isomers, cis-trans isomers, and enantiomers?
Isomers are compounds with the same molecular formula but different structures and properties. Structural isomers have different covalent arrangements of their atoms. Cis-trans isomers have the same covalent bonds but different spatial arrangements (this usually happens when a carbon is double bonded to itself). Cis is if the atoms are on the same side, and trans if the atoms are on different sides. Enantiomers are isomers that are mirror images of each other and usually one will work.
Describe the following functional groups: Hydroxyl
Hydroxyl: OH. They are alcohol, polar, can form hydrogen bonds, and are often found in sugars.

Describe the following functional groups: Carbonyl
Carbonyl: C double bonded to O. Ketones are when the carbonyl group is within the carbon skeleton and there is a carbon on both sides. Aldehydes are when the carbonyl group is at the end of the carbon skeleton and there is a carbon on one side and a hydrogen on the other side. They hydrogen bond and are usually found in sugars.
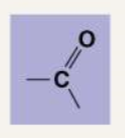
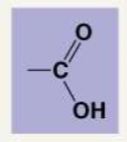
Describe the following functional groups: Carboxyl
Carboxyl: COOH. They are organic acids, and the hydrogen can ionize, leaving behind an oxygen with a negative charge.
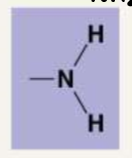
Describe the following functional groups: Amino
Amino: NH2. They can pick up hydrogen and therefore act as a base. If they are ionized, the amino group has a partial positive charge.
Describe the following functional groups: Sulfhydryl
Sulfhydryl: SH. They help cross-link proteins which stabilizes their structure, as they can hydrogen bond with other sulfur molecules. It is found in keratin.

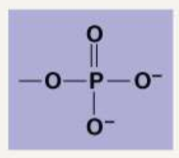
Describe the following functional groups: Phosphate
Phosphate: PO4. They are very electronegative and found in ATP.
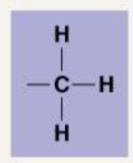
Describe the following functional groups: Methyl
Methyl: CH3. In that region the molecule is nonpolar. They will block enzymes from getting to DNA, which affects the expression of genes. Their arrangement also affects sex hormone shape and function.
What is a calorie?
A calorie is a measure of energy content in food. Carbs and proteins have 4 calories per gram, while lipids have 9 calories per gram. Lipids have more calories, as they have more bonds. It takes 3,500 calories to lose a pound.
What is assimilation?
Assimilation is the incorporation of nutrients into our cells.
What is dehydration synthesis and hydrolysis?
Dehydration synthesis builds polymers by removing a water molecule (hydrogen from one polymer and hydroxyl group from another). Hydrolysis breaks down polymers by inserting a water molecule to break a bond. The number of hydrolysis reactions is one less than the number of monomers.
What is a carbohydrate?
A carbohydrate contains carbon, hydrogen, and oxygen. The oxygen and hydrogen are in a 2:1 ratio. Their monomer is a monosaccharide. They usually have an “ose” ending.
What is carbohydrates' main function?
Carbohydrates’ main function is that they are used for immediate energy and go straight into the bloodstream. This is why they taste sweet.
What are three monosaccharides and three disaccharides? What are glucose monomers held together by?
The three monosaccharides are glucose, fructose, and galactose (they are isomers and are hexoses). Glucose monomers are held together by glycosidic linkages. Maltose is two glucoses and has sugar found in seeds. They have an alpha glycosidic linkage, meaning we have an enzyme to break the bond. Sucrose (table sugar) is glucose and fructose. They have an alpha glycosidic linkage. Lactose (milk sugar) is glucose and galactose. They have beta glycosidic linkages, and not all adults have enzymes (lactase) that can break it.
What is glycogen, amylose, and amylopectin?
Glycogen, amylose, and amylopectin all are stored energy (around four to six hours). Glycogen is animal starch and found in the muscles and liver. They are highly branched and insoluble in water. Amylose and amylopectin are starch found in plants. Amylose is a straight chain, while amylopectin is more branched. They are also insoluble and coil up in water making them ideal for storage. None of these polysaccharides are sweet. We can digest them as we have enzymes that break down the alpha glycosidic linkages.
What is cellulose? What are its glycosidic linkages?
Cellulose is a structural polysaccharide that is found in plant cell walls. It is also known as fiber. It aids in the digestive process, helps lower LDL, and helps regulate blood sugar levels. It has beta glycosidic linkages, so we don’t have an enzyme to break it down. Parallel cellulose monomers are held together by hydrogen bonds between hydroxyl groups. It has no calories.
What are lipids?
Lipids contain carbon, hydrogen, and very little oxygen. They are nonpolar, insoluble in water, and hydrophobic.
What are four types of lipids? Briefly describe each.
Triglycerides include fats and oils. They are used for stored energy, and as they are stored in the adipose tissue (under the skin but over the muscle), they provide heat, insulation, and padding. Phospholipids help with cell membrane structure. Steroid hormones are made from cholesterol and are involved in chemical signaling. Waxes make a protective coating from water on the surface of leaves and fruits. They are a hydrophobic barrier that prevents transpiration.
What are triglycerides made of? What is their bond called?
Triglycerides are made of glycerol and three fatty acids. Their bond is an ester bond. The max number of dehydration synthesis reactions for a triglyceride are three.
Describe saturated fatty acids.
Saturated fatty acids have single carbon-carbon bonds, and they have as many hydrogen atoms as possible. They are solid at room temperature and usually come from animal sources (except coconut oil which is also saturated).
Describe unsaturated fatty acids. Make sure to talk about cis and trans fats.
Unsaturated fatty acids have one or more double bonds along the carbon chain (it is not saturated with hydrogen). This creates a bent shape. If it is monounsaturated, there is one double bond. If it is polyunsaturated, there are two or more double bonds. It is liquid at room temperature and usually from plant sources. If it is a cis fatty acid, the hydrogens are on the same side and it’s healthy. Trans fats are made through hydrogenation which adds hydrogen to liquid vegetable oils to make them more solid. Trans fats are even more stable than saturated fats, so our body breaks them down last (or never). This can clog up our arteries, raise LDL, and even lower HDL (unlike saturated fatty acids). They are usually found in commercially fried or baked goods.
Which is better for the body: saturated or unsaturated fat?
Unsaturated fats are better than saturated fats, as they are metabolized much faster, they don’t leave fatty streaks or plaques in our arteries as they’re liquids and they lower LDL.
What is cholesterol?
Cholesterol is a soft waxy substance made in the liver and consumed in our diet. They make steroid hormones, and they maintain the fluidity of the cell membrane. They are found in animal membranes. Cholesterol has four carbon rings, and then hormones add functional groups to that.
What is atherosclerosis?
Atherosclerosis is the hardening of the arteries due to the accumulation of cholesterol and saturated fats, which limits blood flow. It can lead to hypertension (high blood pressure), a heart attack, or a stroke.
What are phospholipids?
Phospholipids make up a majority of the cell membrane. They are composed of glycerol, two fatty acids, and one phosphate group. They are amphipathic, as the phosphate group is polar, but the fatty acids are nonpolar. In the phospholipid bilayer, the hydrophobic tails face each other.
What are proteins? How do plants get one of its elements?
Proteins contain carbon, hydrogen, oxygen, and sometimes sulfur. Plants get nitrogen by absorbing it from the soil through capillary action and then assimilating it into their tissue.
What is a protein monomer? How many are there? What does the “R” group determine?
A protein monomer is an amino acid. There are twenty different ones. The “R” group is the variable side chain. It gives the amino acids its chemical properties such as polarity and if it is acidic or basic. Cysteine is an amino acid that has sulfur. It is very strong and bonds to itself by forming disulfide bridges.
What bond connects amino acids? What are two amino acids? Three amino acids?
Peptide bonds connect amino acids. Two amino acids are dipeptides, and three amino acids are tripeptides.
What is the primary structure? Secondary structure? Tertiary structure? Quaternary structure?
Primary structure is determined by the order of nucleotides and is a specific sequence of amino acids. They are stabilized by peptide bonds. Secondary structure is when the polypeptide is coiled into an alpha helix or folded into a pleated sheet. The “R” groups are not involved but rather the carboxyl and amino ends are. They are stabilized by hydrogen bonds. Tertiary structure is the overall 3-D shape, and it results from the interactions among the “R” groups. For example, polar is outside the polypeptide, and then the inside is nonpolar. Covalent bonds form between the sulfhydryl groups to stabilize proteins. Quaternary structure is when proteins are made up of 2 or more polypeptide chains/subunits. Hemoglobin has 4 polypeptides.
What is denaturing? How is this different from genetic mutations?
Denaturing is when a protein changes its shape and can no longer function. Sometimes a protein can renature. This affects the secondary, tertiary, and quaternary structure, but not the primary structure. Genetic mutations can cause non-functional proteins. It changes the primary, tertiary, and quaternary structure, but not really the secondary structure.
What three things can impact denaturation?
Temperature, pH, and salt impact denaturation. The temperature should be around 37 degrees Celsius. If it’s too hot, the protein can denature. If it’s too cold, the protein will slow down in function but not denature. The pH should be around 7 (blood is 7.4 and stomach acid around 1-2). Salt should be around 1-2%, as the ions/charges could attract the protein, pulling it out of shape. This can be seen in perming.
What are the functions for proteins?
Proteins have many functions. As enzymes, they act as organic catalysts by speeding up chemical reactions by lowering the activation energy. They are specific and can be reused. Motor and contractile proteins include actin, myosin, microfilaments, and the movement of the cilia and flagella. They require ATP and dynein motors. They are involved in immune defense as antibodies attack antigens. Proteins like hemoglobin are transport proteins that carry molecules into and out of the cell membrane (transmembrane proteins) or throughout the body. Structural proteins include collagen, keratin, and microtubules (made of tubulin). They usually have the cysteine amino acid. Storage proteins are used for tissue growth such as casein, protein of milk, and ovalbumin, the protein of egg white. Lastly, they are used for hormones and signaling which allows for the coordination of an organism’s activity. Insulin regulates sugar in the bloodstream. Receptors in a nerve cell detect signaling molecules from other nerve cells.
What are nucleic acids?
Nucleic acids contain hydrogen, oxygen, carbon, phosphorus, and nitrogen. They are the blueprints for proteins.
What is deoxyribonucleic acid vs ribonucleic acid?
Deoxyribonucleic acid (DNA) is found in the nucleus of every eukaryotic cell, contains our genes (specific order of nucleotides), and codes for the physical traits and metabolic functions of an organism. Ribonucleic acid (RNA) reads the genes and synthesizes proteins. Every three bases is a codon, which codes for one amino acid. There is messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA).
What is the monomer of a nucleic acid?
The monomer of a nucleic acid is a nucleotide. It has a phosphate group, 5-carbon sugar, and nitrogenous base. Its polymer is a polynucleotide.
What are the nitrogen bases for DNA? Which are pyrimidines vs purines? What is the different nitrogen base in RNA?
The nitrogen bases for DNA are adenine which bonds with thymine and guanine which bonds with cytosine. Cytosine and thymine are pyrimidines, meaning they have one ring. Guanine and adenine are purines, meaning they have two rings. Adenine and thymine form two hydrogen bonds, while guanine and cytosine form three. This means it takes more energy to separate amino acids and cytosine. In RNA, uracil replaces thymine.
What holds the sugar-phosphate backbones together? What about the nitrogenous bases?
Phosphodiester bonds hold the sugar-phosphate backbones together. The nitrogenous bases are held together by hydrogen bonds.
What does it mean when DNA is antiparallel?
DNA is antiparallel so each strand goes in opposite directions (one 5’ to 3’ and the other 3’ to 5’). The side that is 5’ ends with a phosphate group.
What is transcription and translation?
Transcription is the process that copies DNA into RNA. Translation is the process that reads the RNA and translates the message into amino acids.
What are mutations? Give two examples
Type 1 Diabetes has a mutation in the DNA coding for insulin, which it doesn’t produce. This is bad, as insulin is required for blood glucose regulation.
Sickle cell disease is a mutation in the DNA coding for hemoglobin. Instead of a globular shape, it has a sickle cell shape. It gets stuck in the capillaries, which limits blood flow, meaning there is less oxygen delivery. This leads to extreme fatigue, pain, and organ damage.