(a) energetics
1/10
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
chemical reaction in which heat energy is given out (3.1)
exothermic
chemical reaction in which heat energy is taken in (3.1)
endothermic
calorimetry experiment for combustion (3.2)
calorimetry experiment for displacement (3.2)
calorimetry experiment for dissolving (3.2)
calorimetry experiment for neutralisation (3.2)
equation for heat energy change (3.3)
Q (measuring in kJ) = mcΔT
equation for molar enthalpy change (ΔH) (3.4)
Q / mol
Q is measured in kJ, so ΔH is measuring in kJ/mol
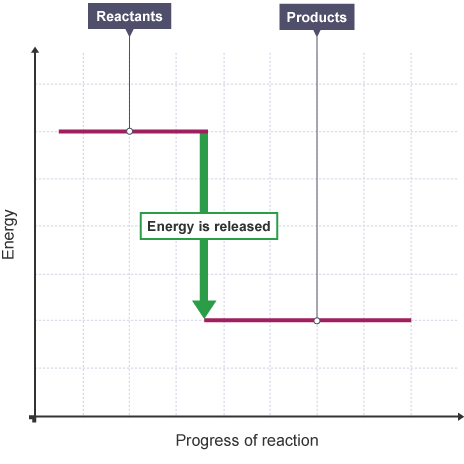
energy level diagrams for an exothermic reaction (3.5C)
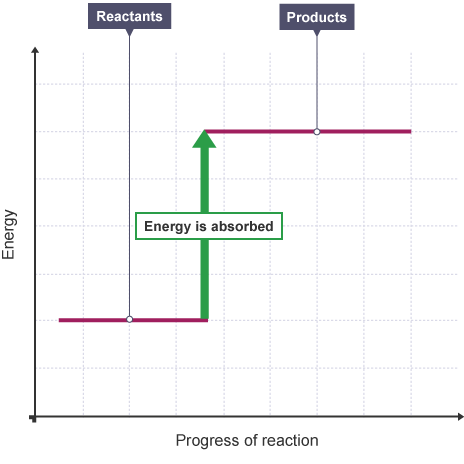
energy level diagrams for an endothermic reaction (3.5C)
bond-breaking and bond-making (3.6C)
bond-breaking is an endothermic process (taking in energy) because the energy needed to break the bonds is more than the energy released in making new bonds
bond-making is an exothermic process (giving out energy) because the energy needed to break the bonds is less than the energy released in making new bonds