Reactions (alkane based)
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
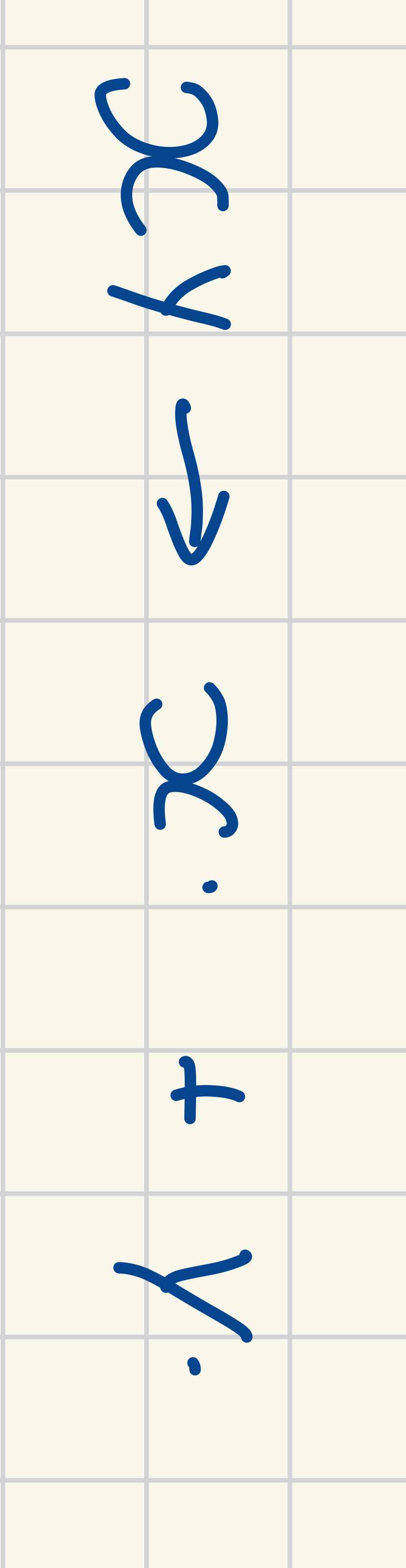
Bond fission: homolytic
The bonds break evenly and each bonding atom receives one electron from the bonded pair = free radicals
2
New cards
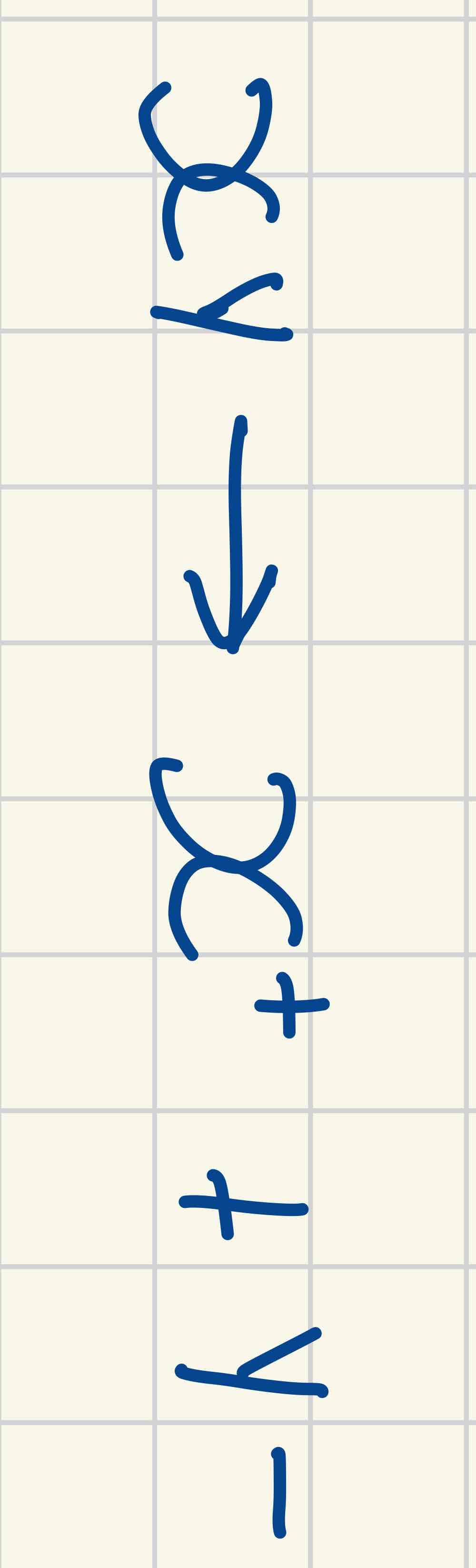
Heterolytic fission
Bonds break unevenly one of the bonded atoms receive the pair of electrons producing ions
3
New cards
Curly arrow
Shows the movement of pair of elections
4
New cards
Free radical substitution.

5
New cards
Problems with free radical substitution
You can get mixture of products
To combat this → have excess of reactant that isn't the halogen
Can get a mixture of isomers as the reaction can take place at any point of the chain
6
New cards
Catalytic cracking
Turns long hydrocarbon chain into smaller alkane and alkene
7
New cards