Topic 6: Atmospheric Systems and Societies
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
atmosphere
gases surrounding the earth
dynamic systems
characteristics of a system can chnage over time
troposphere
the lowest layer of the earth atmosphere
stratosphere
the second lowest layer of the earths atmosphere
cloud albedo
clouds reflect sunlight from transferring heat to the earth’s surface therefore cooling the area bellow
ozone
O3 molecules that are naturally found in the stratosphere
ozone layer
O3 molecule layer that helps the absorption of harmful UV rays (UV3/some UV2) and allows UV1 to pass through for plants, animals and humans health benefits
Ultraviolet Radiation (UV radiation)
non-ionsising radiation eiitted by the sun
Ozone-Depleting Substances (ODS)
chemicals that destroy the ozone layer
CFCs
non toxic, non-flammable chemicals containing atoms of carbons, chlorine and fluorine
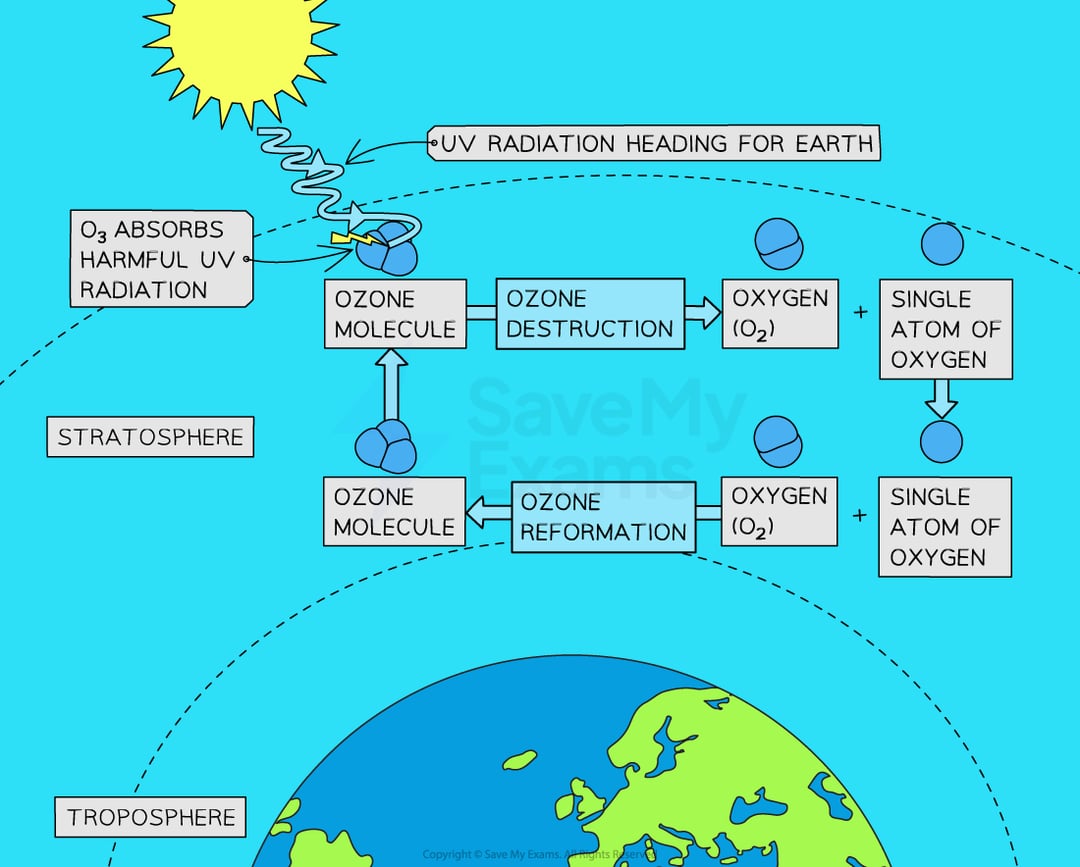
how is stratospheric ozone formed?
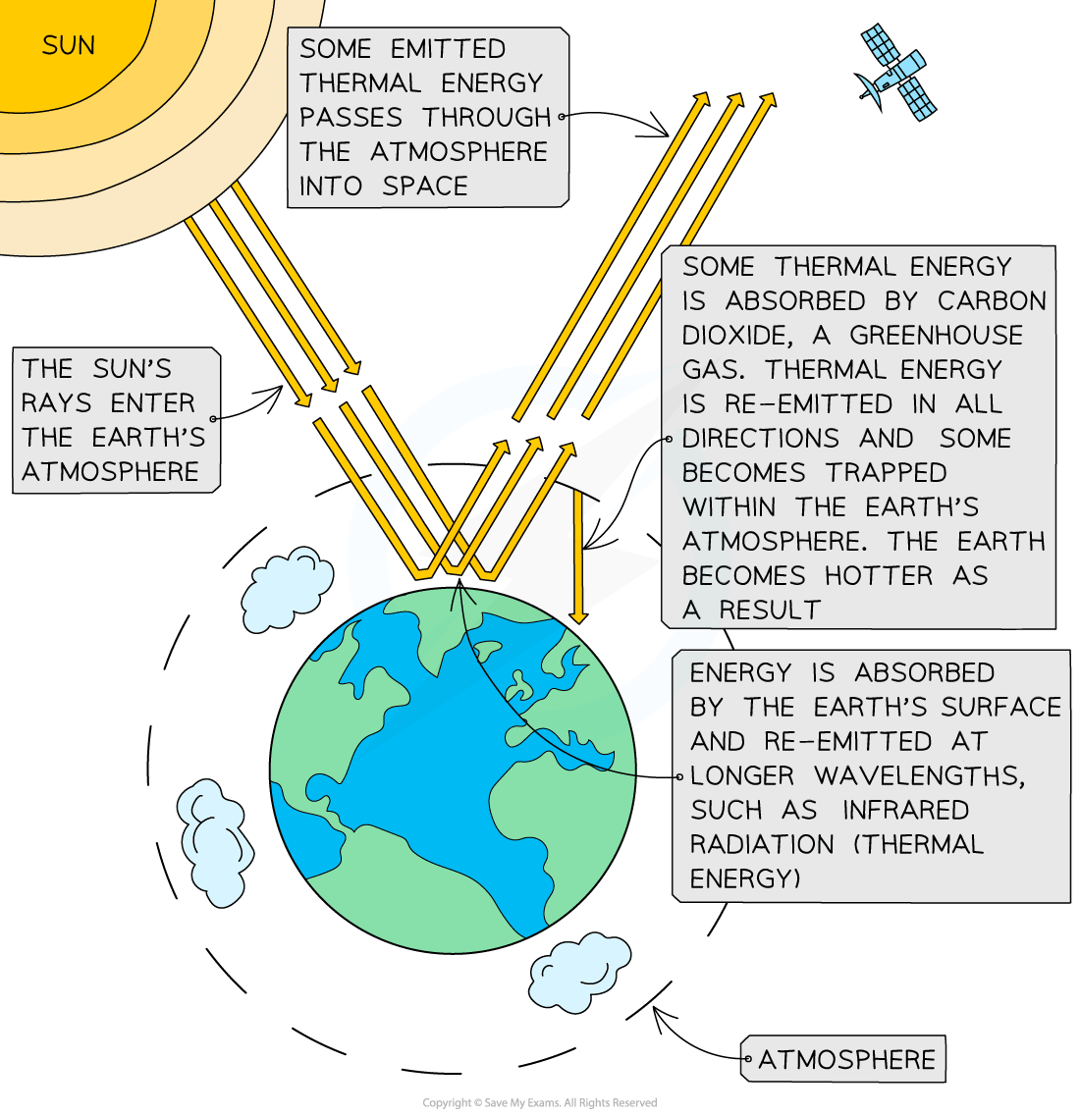
describe the greenhouse effect
what are the effects of UV2 rays?
increased mutation rates in DNA causing cancers
cataracts
damages a plants ability to photosynthesise
reduces primary production
how do ODS break down ozone?
when released into the atmosphere, they arrive in the statosphere and the energy transfere from UV light causes the release of the halogen that then reacts with ozone to take one molecule of oxygen
what happened to CFCs?
the Montreal Protocol - CFCs were no longer legally produced or consumed in may industrialised coutries
what are the ODS found in?
refrigerants
propelants
gas blow plastics
methyl bromide
evaluate ozon management strategies
illegal trade of CFCs was found
HCFCs were introduced as a substitute but are a strong greenhouse gas
some ozone depletion can occur from natural disasters such as volcanic eruptions
non-aerosol alternatives can be used but not promoted as a benefit to the atmosphere
primary pullutant
pollutant emitted directly from the source s
secondary pollutant
formed when other pollutants react in the atmosphere
photochemical reaction
occurs when light energy is absorbed by a substances molecules
particulates
microscopic solids or droplets that can be inhaled
smog
primary and secondary pollutant mixture
thermal inversion
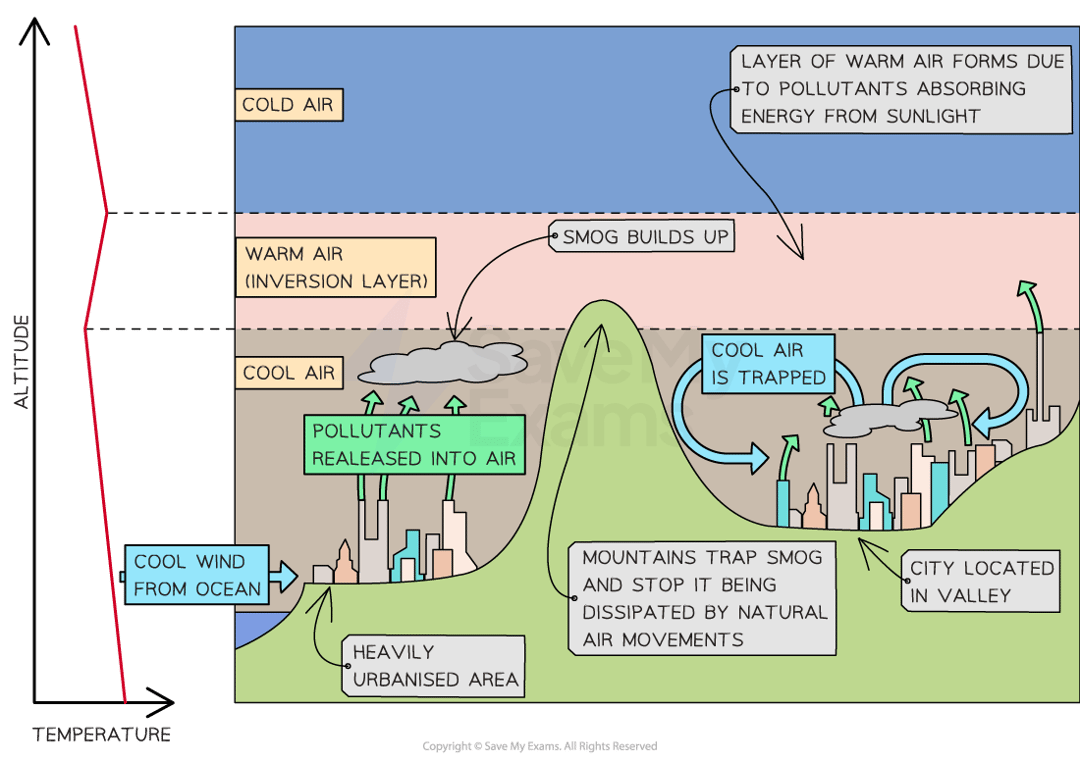
warm air is trapped between two layers of cold air
tropospheric ozone
formed as a secondary pollutants by reactions involving nitrogen oxides. NOxs are formed from oxygen and nitrogen in internal combustion, usually forming nitrogen monoxide and nitrogen dioxide that then reacts with sunlight to form NO + O to which the O reacts with O2 to form O3. this can also react with NO to from NO2
what affects the amount of smog in a cit?
the amount of people and their vehicles
to topography - it can trap smog if the city is surrounded by uplands or mountains
weak winds - the smog cannot be dispersed
thermal inversion
forest fires
industrial factories
catalytic converter
they convert the harmful pollutants into harmful pollutants before release but they decrease fuel efficiency and increase CO2 emissions
wet desposition
rain, slee, ssnow,fog that has become more acidic that nrmal
dry deposition
gases, durst that has become more acidic
lichens
organisms that grow in exposed places such as rocks, tree and bark such as fungi, algae and microbes, they can be used as SO2 level indicators as they suffer a lot from SO2 exposure
liming
when material from dissolved rocks enter the soil
waste gas scrubbers
waste gas treatment where gas stream is put in contact with a liquid to allow certain gases to pass into the liquid