RESPIRATORY & CARDIORESPIRATORY INTEGRATION
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Identify the three phases of respiratory rhythm
inspiration
post inspiration
late expiration
Describe inspiration including the nerves and muscles that are activated
Inspiration:
Diaphragm contracts, activated by the phrenic nerve, expanding the thoracic cavity (also intercostal muscles fire)
The recurrent laryngeal nerve (vagus nerve) activates the abductor muscles of the upper airways to contract, reducing subglottal pressure
Thoracic cavity and airway expands → intrapulmonary pressure decreases below atmospheric pressure → air flows into lungs
Describe post-inspiration including the nerves and muscles that are activated
Post-Inspiration:
The phrenic nerve activity stops, relaxing the diaphragm
The recurrent laryngeal nerve increases its activity even more, activating the adductor muscles, constricting the upper airways
Increased subglottal pressure → slows the outflow of air → increases diffusion as air can remain in lungs for slightly longer
note: this phase is when most modulation occurs (e.g. when talking)
Describe late expiration including the nerves and muscles that are activated
Late Expiration
Both nerves have very little activity, relaxing the diaphragm, upper airway and intercostal muscles
This increases the rate of air outflow, enabling intra-alveolar pressure to return to atmospheric pressure and the intra-plural pressure to return to resting
This increases the rate of outflow of air
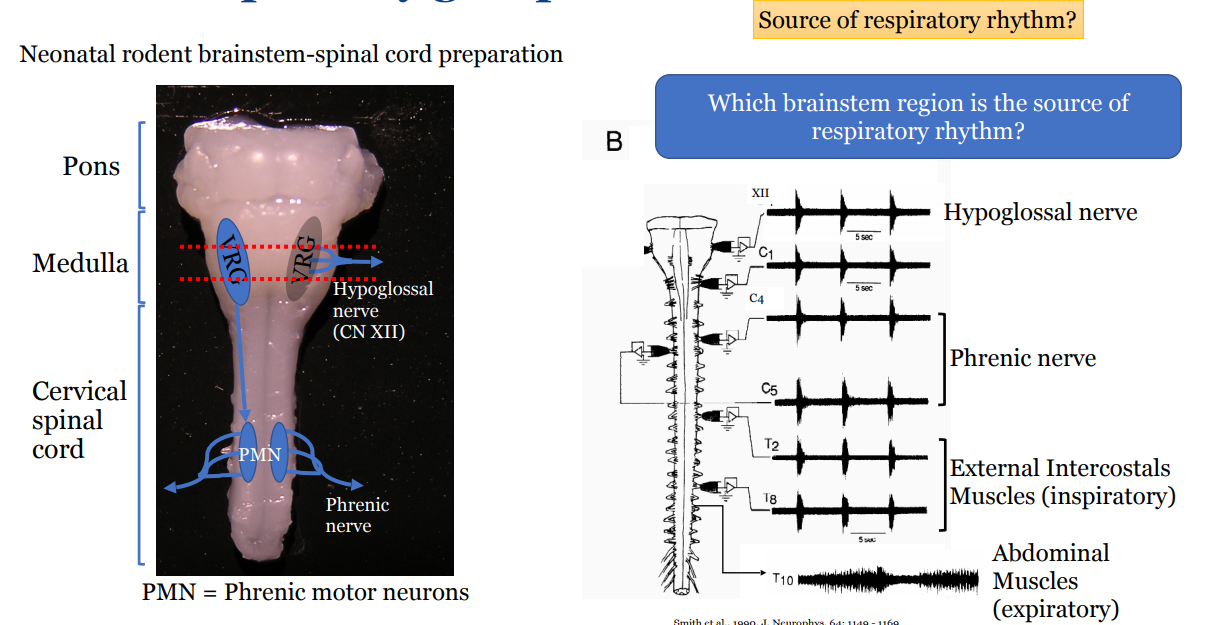
Describe the process by which the pre-Bötzinger complex was identified as the potential respiratory rhythm generator
Found synchronous activity in a number of nerves but wanted to locate the source of the respiratory rhythm
Explored by slicing sections of the brainstem and measuring the pattern of response
Slicing where we now know is the VRG, caused respiratory rhythm of all nerves to disappear
thus this is the area that controls respiratory rhythm
To learn specifically where: looked at and recorded the sagittal section of the rat
Cells associated with inspiration were found on the pre-Bötzinger complex that aligned with the respiratory rhythm
Blocking the activity of neurons in this complex also lead to the loss of respiratory rhythm
Describe how Pre-BötC neurons can be characterised functionally
Pre-BötC are pre-inspiratory neurones
Little to no expiratory neurones
Glutamatergic
Subset that is glycinergic
Thus is functionally excitatory and inhibitory
Describe how Pre-BötC neurons can be characterised chemically
There are no unique markers that define these neurones
NK1-receptor expressing
Somatostatin (SST) expressing
Dbx-1 (transcription factor) expressing
None of these markers are exclusive to this complex, so when these are expressed, it is only likely that they are part of this complex
Describe how Pre-BötC neurons can be identified
Selective lesion of > 80% of NK1 receptor expressing cells in Pre-BötC using Saporin-Substance P
Lead to irregular breathing patterns with large periods of apnea
Thus NK1-R expressing cells in Pre-BötC are required for normal breathing rhythm generation
Confirmed more recently, using allatostatin:
allatostatin inhibits the Pre-Bötzinger Complex, which causes breathing to stop, meaning it is essential for respiratory rhythm generation
Describe the properties of the Pre-Bötzinger complex
Pre-Bötzinger neurons are pacemakers that have intrinsic bursting properties
if you block neurotransmitter receptors, action potentials of these cells still continue
The intrinsic bursting properties are reliant on persistent sodium currents in the Pre-BötC neurones
if you block sodium currents (through riluzole), the rhythmic bursts stop
Also reliant on calcium ion activated non-selective cation currents
if you block calcium activated non-selective cation currents there are a smaller intrinsic bursts
What happens if you destruct or silence neurons in the Pre-BötC?
Destruction or silencing of neurons in the Pre-BötC leads to severe disruptions in respiratory rhythm including apnea and altered breathing, demonstrating the critical role of these neurons in generating normal breathing.
Describe a key characteristic of the Pre-BötC in terms of activity generation
Respiratory rhythm is an emergent property
In an experiment it took 9 small bursts it takes to get a big burst of activity
Identify briefly evidence from humans that supports the importance of the Pre-BötC in respiratory function
Looked at the Pre-BötC in humans and found:
the number of cells in the pre BötC is much smaller for someone with multiple system atrophy (which is sleep apnea and dysregulated breathing)
Identify other rhythmogenic sites and explain the current view of respiratory rhythm generation
Pre-Bötzinger Complex drives inspiration and is still the pacemaker, interacting with both other complexes to set the base respiratory rhythm:
PiCo (post inspiratory complex)
pFL (parafacial nucleus)
whether these complexes communicate is not clear
RTN/pFRG activation drives active expiration
Describe the organisation of the respiratory neural network
The respiratory neural network is organized into distinct rhythmic centers, including the Pre-Bötzinger Complex, the Bötzinger Complex, and the Pons, which coordinate and modulate the rhythmic pattern of breathing through complex interactions.
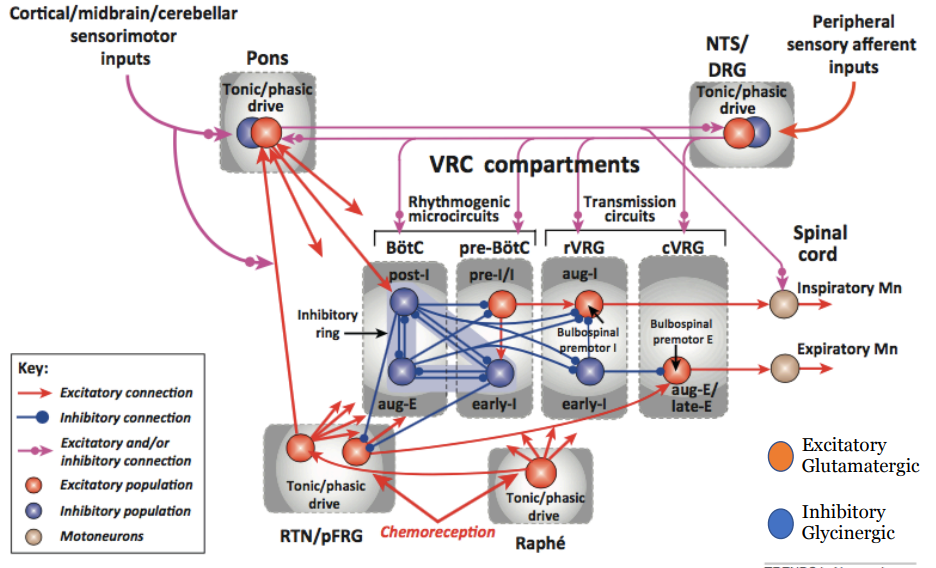
Identify the sub-regions of the ventral respiratory group (VRG)
There are four different regions of the VRG
From rostral to caudal:
BötC
pre-BötC
rVRG
cVRG
Describe the different types of neurons within each region of the VRG
There is some topographical organisation of the respiratory neurons
Within the BötC, we have post-inspiratory and augmenting expiratory neurons (as one fades the other picks up through post-inspiratory and late expiratory phases)
These tend to be inhibitory glycinergic
help terminate inspiration and reinforce expiration phase (respectively) by inhibiting inspiratory neurons and activity
In the pre BötC, there are pre-inspiratory neurons (excitatory glutaminergic) and early inspiratory neurons (inhibitory)
decrease in activity over the period of inspiration
trigger the onset of inspiratory bursts and shape the pattern of inspiratory activity
In rVRG there are augmenting inspiratory (excitatory to motor outputs)
increase in activity over the period of inspiration
drive motor output to diaphragm and intercostals
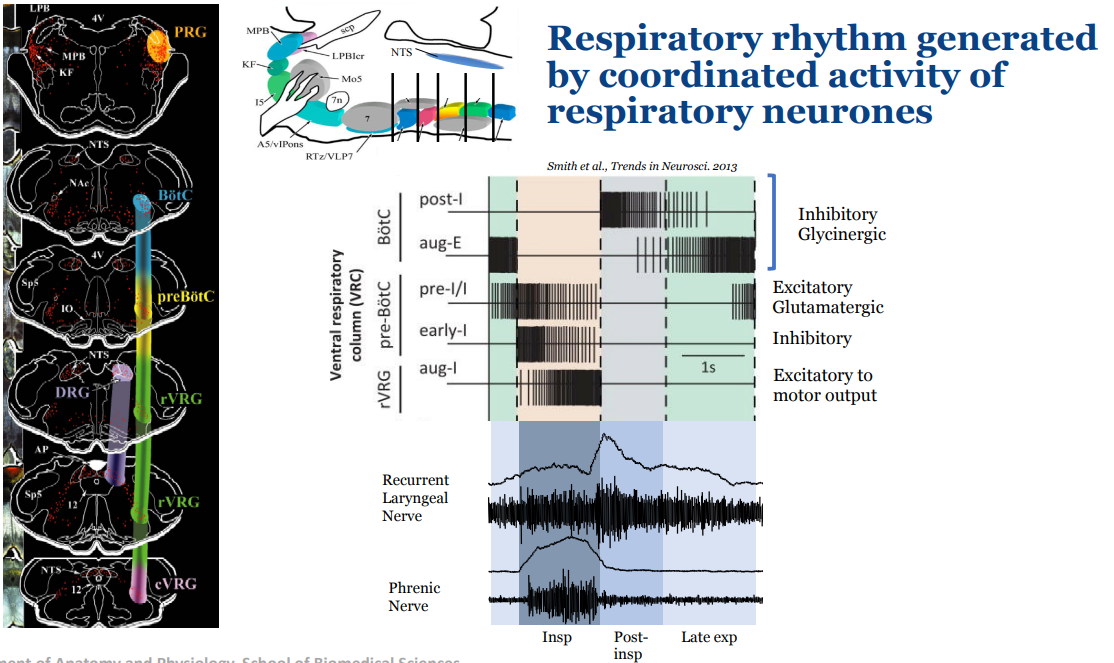
What happens if you inject DLH to the Pre-BötC and BötC?
Injecting DLH = glutamate receptor agonist
If you excite the BötC it will excite expiratory neurons, extending the expiratory phase
If you excite the Pre BötC it will excite pre-inspiratory neurons, initiating the rhythm bursts sooner
What happens to respiratory rhythm if you activate different regions within the respiratory network?
Pre-BötC initiates and drives inspiratory bursts.
PiCo is activated slightly after inspiration and shapes post-inspiratory airflow by slowing expiratory recoil.
RTN/pFRG becomes active during metabolic/physical demand, recruiting active expiration.
BötC provides inhibitory control, terminating inspiratory drive and regulating transitions between phases.
What are the three important regions that generate the respiratory rhythm?
Pontine respiratory group important for post-inspiratory phase
BötC important for expiratory phase
Transection caudal of the pre-BötC abolished respiratory rhythm
Remove pre-BötC = a short decrementing pattern (only inspiratory phase too)
Describe how the three phase rhythm looks from a nerve activation perspective
The three phase rhythm which is most clearly identified in the cervical vagus nerve (cVN), which is the main nerve of the recurrent laryngeal nerve (RLN)
Inspiration defined by the phrenic nerve
Post inspiration which is the decrementing component of the recurrent laryngeal nerve
Short phase called late expiration (which is short in this case) that is defined by then the recurrent laryngeal nerve has almost no activity to when the phrenic nerve activity starts
The hypoglossal nerve activity occurs pre inspiration to ensure the tongue and upper airways are open before the phrenic nerve is active
What happens if you remove the pontine respiratory group?
Pontine respiratory group important for post-inspiration
The pons provides an excitatory drive to post-i neurons in the BötC
the bursting is still there
post-inspiratory phase burst is lost
Inspiration and expiration are longer, and it has changed into a two phase rhythm
What changed most is the cVN activity, the timing of the burst is now synchronised with the phrenic nerve, however there is now no more big burst after the phrenic burst
Identify scenarios when respiratory rhythm is modulated from the basal rhythm
When talking, exercising etc
Describe how pulmonary stretch receptors can modulate respiratory rhythm
Pulmonary stretch receptors are sensory receptors located in the lung's smooth muscle that help regulate the breathing pattern by detecting lung inflation. When activated, they send signals to the brain to inhibit further inspiration, thus preventing overinflation of the lungs.
Activation of PSRs will result in what changes in the respiratory phases?
Inhibition and termination of inspiration
Slowing of respiratory rhythm
Prolongation of expiration
What will the effect of activation of PSRs be on respiratory neurons?
Inhibit pre-inspiratory neurons
Inhibit inspiratory neurons
Excite post-inspiratory neurons
Inhibit augmenting expiratory neurons
Describe the chemoreceptor response to hypoxia
The glomus cells (type I), located in the carotid bodies and aorta, detect low oxygen levels (hypoxia) in the blood (but also high CO2 and glucose levels)
Glomus cells get activated by depolarisation of the cell membrane through different mechanisms such as inhibition of potassium leak channels
they transmit their signals via paracrine actions (using ATP) to the glossopharyngeal nerve which terminates at the NTS, activating the autonomic nervous system to increase respiration and cardiac output
Hypoxia response is to increase sympathetic nerve activity which manifests through an increase in HR and a small increase in BP
Describe the location of central and peripheral chemoreceptors
Peripheral Chemoreceptors
Location: Carotid bodies (mainly) and aortic bodies
Central Chemoreceptors
Location: Retrotrapezoid nucleus (RTN)
Describe how hypercapnia stimulates RTN neurons
CO2 that's in the blood will enter the capillaries within the brain, diffuse across the BBB into the CSF where it undergoes a chemical reaction with water creating hydrogen ions
The hydrogen ions activate the RTN neurons through activating TASK-2 (ion channel) and GPR4 (GCPR) channels, increasing neuronal activity through an unknown mechanism
astrocytes also increase the activity of RTN neurons
Increase in activity of RTN neurons will increase breathing and sympathetic nerve activity
It will also evoke active expiration (through aug-e excitation) during late expiratory phase
Breaths become bigger and the abdominal nerve now has bursts
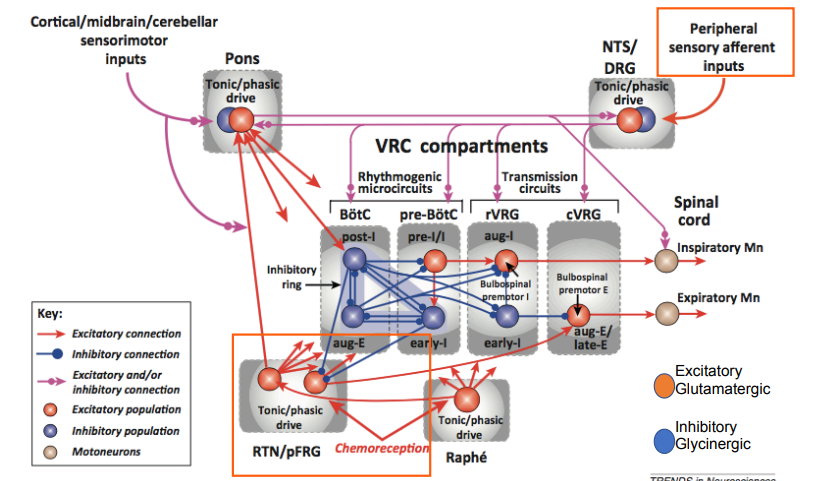
Provide an overview of the modulation of the respiratory rhythmogenic network by peripheral afferent inputs and central chemoreceptors
The respiratory rhythmogenic network is modulated by peripheral afferent inputs from chemoreceptors detecting changes in blood O2 levels and by central chemoreceptors responding primarily to CO2 levels.
Peripheral chemoreceptors respond to low oxygen in the blood
Central chemoreceptors respond to high carbon dioxide in the cerebrospinal fluid
Peripheral inputs, such as those from carotid and aortic bodies, synapse onto the NTS which projects to the VRG, enhancing respiratory drive through an increase in sympathetic activity
Central chemoreceptors in the RTN respond to hypercapnia, altering breathing patterns (switching from tonic to phasic) and increasing sympathetic activity
activates aug-e neurons that drive the expiratory motor neurons for expiratory muscle activity
Describe how muscle contraction and exercise modulates respiratory rhythm and pattern and how is this mediated?
There is a 1:1 coupling between step frequency and breathing
Muscle contractions = somatic afferent stimulation
Afferent feedback from contracting muscles resets respiratory rhythm
Stimulation of somatic afferents leads to shortening of post-I phase due to inhibition of Post-I neurons (in the BötC) + excitation of aug-E neurons
This shortens expiration and leads to earlier inspiration
The rhythm resets at the next inspiration, which is mediated by the PRG, which is mediated by substance P in the BötC
What does resetting result in relating to the respiratory rhythm
Resetting results in early onset of inspiration
occurs with shortening of expiration by inhibition of post-inspiration
Describe the relationship between sympathetic activity and respiration
Sympathetic Nerve Activity and Heart Rate are Modulated by Respiration
Describe what happens to the relationship between sympathetic activity and respiration during hypertension
It is altered
Altered respiratory sympathetic interaction is a driver for the development of hypertension
In a hypertensive animal, the sympathetic activity is much more associated - the bursts are bigger and everything seems to be phase locked to respiration
In a normotensive animal, more sympathetic nerve activity occurs between bursts and the bursts aren't as large
Describe how the sympathetic respiratory coupling interaction occurs
The heart is in the thoracic cavity so is subject to sudden thoracic pressure changes, impacting venous flow and heart rate
This is one way that breathing impacts heart rate
There is another reflex that links respiratory rate to heart rate, the Hering-Brauer Reflex
When you breathe in, the stretch receptors increase in activity and send information via the NTS, changing the activity of sympathetic nerves impacting both respiration and HR
This relationship between respiration and sympathetic activity is totally reliant on the output of the c1 neurons in the RLVM which are recieving respiratory input
What is the relevance of the respiratory coupling in terms of hypertension?
Sympathetic activity is time-locked with respiration, particularly in hypertensive rats. The resulting bursts of activity result in increased noradrenaline release and NPY co-transmission in postganglionic sympathetic neurons.
Explain how hypertension occurs in relation to respiratory coupling and how this is relevant in treatment
When respiratory modulation is exaggerated, it leads over time to blood pressure escaping from homeostatic control, being elevated, and hypertension (seen through amplified Traube-Hering waves)
If we can identify these people, if we inhibit the C1 neurons, we can reverse this phenotype, stopping the development of the hypertensive phenotype
The C1 neurons being catecholaminergic, there are drugs that are relatively selective at inhibiting these types of cells