OCR Chemistry A-level Module 3
1/113
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
114 Terms
Enthalpy change
the amount of energy absorbed by a system as heat during a process at constant pressure
Thermodynamics
the branch of physical chemistry that focuses on energy in a chemical system and how it can be changed into other forms of energy
Enthalpy
the heat content of a system
Enthalpy change of formation
The energy change that takes place when 1 mole of a compound is formed from its constituent elements in their standard state under standard conditions
Enthalpy change of combustion
The energy change that takes place when 1 mole of a substance is completely combusted
Enthalpy change of neutralisation
The energy change associated with the formation of 1 mole of water from a neutralisation reaction.
specific heat capacity
the energy required to raise the temperature of one gram of a substance by one kelvin
Calorimetry
The quantitative study of energy change in a chemical reaction
How is the periodic table arranged
-Arranged by increasing atomic number
-In periods showing repeating trends in physical and chemical properties (periodicity)
-In groups having similar chemical properties
Definition of first ionisation energy
removal of 1 mole of electrons from 1 mol of gaseous atoms
Metallic bonding
Strong electrostatic attraction between cation(positive ions) and delocalised electrons
Explain the metallic lattice structure
Metals have giant metallic lattice structures held together by metallic bonds
Explain the solid giant covalent lattice of carbon (diamond, graphite and graphene) and silicon
networks of atoms bonded by strong covalent bonds
Giant metallic lattices properties (MP, BP, solubility and electrical conductivity)
They are good conductors because of the delocalised electrons and have a high melting and boiling point as it takes a lot of energy to break the electrostatic attraction
Giant covalent lattices properties (MP, BP, solubility and electrical conductivity)
Giant covalent have high boiling and melting points. Most are poor conductors as there are no delocalised electrons or charged ions roaming freely
Use of calcium hydroxide
Used in agriculture to neutralise acid soils
Physical properties of halogens
-They exist as diatomic molecules
-Boiling point of cl, br and 1 in terms of london forces
Definition of disproportionation
Is the oxidation and reduction of the same element
Examples of disproportionation reactions
Chlorine and water (used in water treatment)
-Chlorine with cold dilute aqueous sodium hydroxide (used as a form of bleach)
Benefits of chlorine use in water treatment and the risks
-Kills bacteria
-toxic hazards of chlorine gas
-Chance of formation of chlorinated hydrocarbons
What is activation energy definition
Minimum energy required for a reaction to take place
Enthalpy change of formation definition
The formation of 1 mol of a compound from its elements
Enthalpy change of combustion definition
The complete combustion of 1 mol of a substance
Enthalpy change of neutralisation definition
Formation of 1 mol of water from neutralisation
What are standard conditions?
298K and 100kPa
Enthalpy change of reaction
Enthalpy change associated with a stated equation
What is average bond enthalpy?
Breaking 1 mol of bonds in gaseous molecules
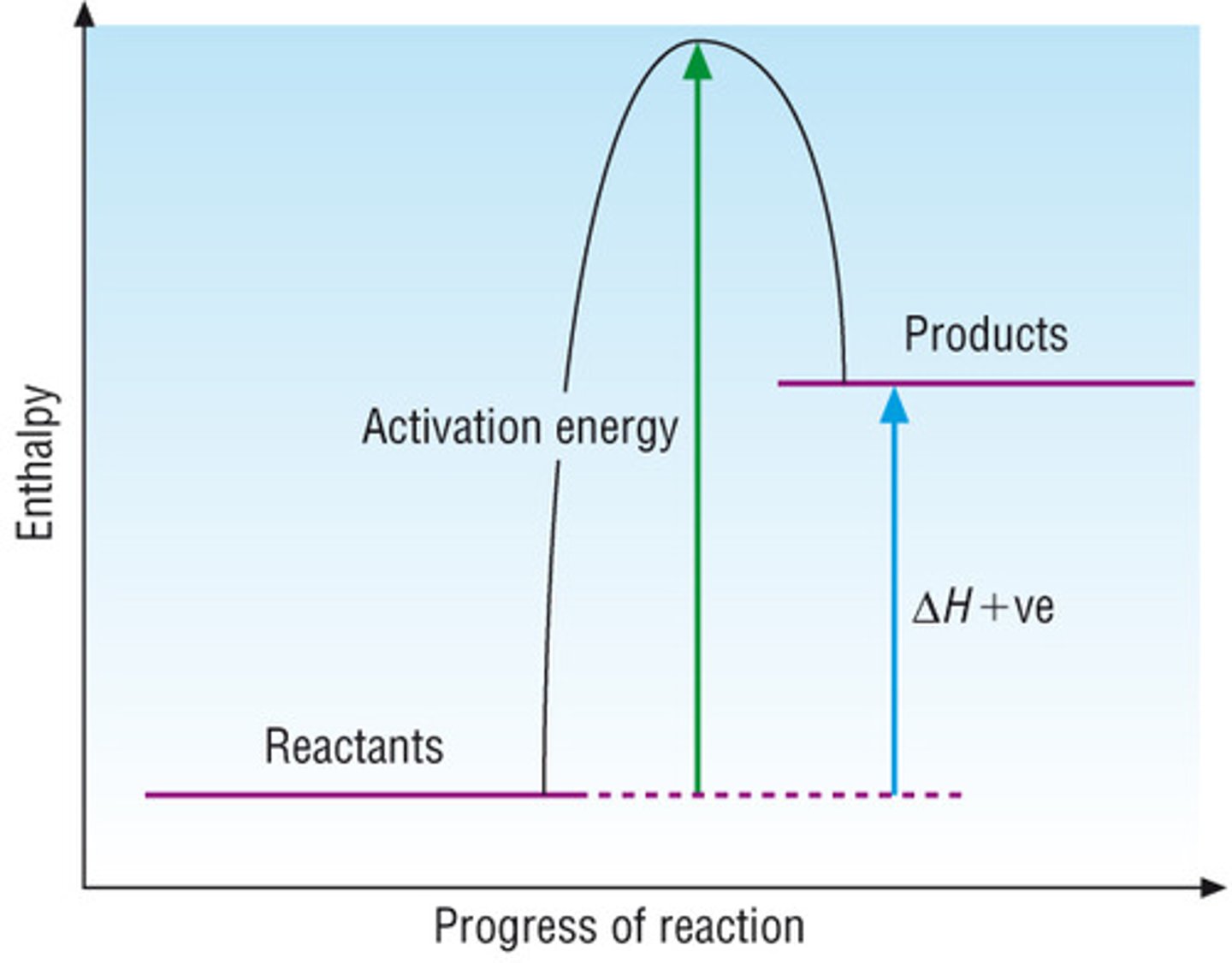
What is an endothermic reaction?
In an endothermic reaction, the reactants have less enthalpy than the products. Thermal energy is changed into chemical and energy is gained by the chemicals and lost by the surroundings which decreases the temperature
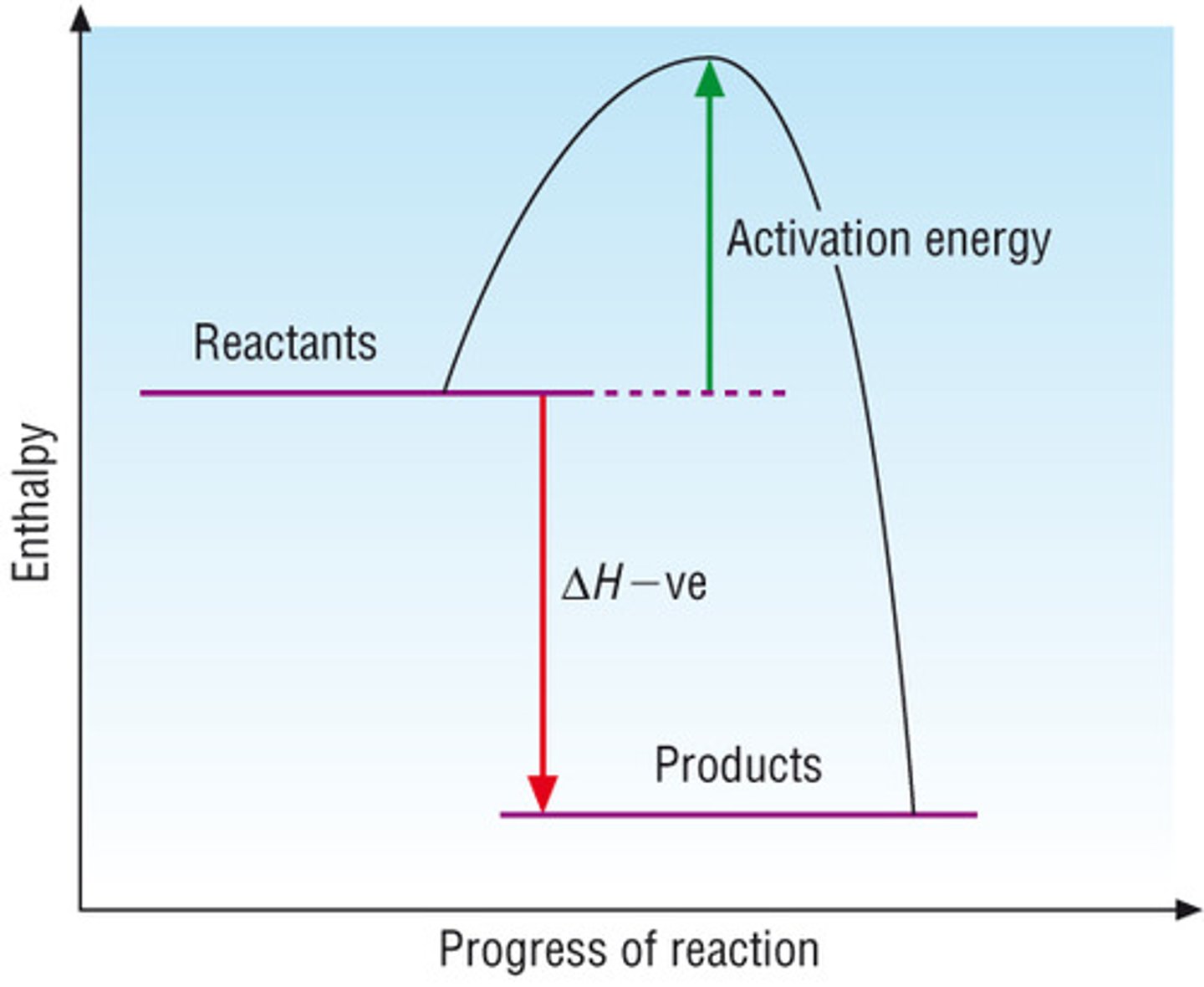
What is an exothermic reaction?
In an exothermic reaction, the reactants have more enthalpy than the products. Chemical energy is changed into thermal energy and energy is lost by the chemicals and gained by the surroundings which increase the temperature of the atmosphere
What does a catalyst do?
Increases reaction rate without being used up by overall reaction by allowing a reaction to proceed via a different route with lower activation energy, as show by enthalpy profile diagrams
What is a homogenous catalyst?
A homogenous catalyst is in the same state as the reactants such as Cl radicals acting as a catalyst for the breakdown of the ozone
What is a heterogenous catalyst?
A heterogeneous catalyst is in a different state from the reactants. An example would by the Harber process which is catalysed by Iron
Economic importance of and benefits of catalysts
-Increased sustainability by lowering temperatures
-Reducing energy demands from the combustion of fossil fuels resulting reduction in CO2 emissions
Techniques and procedures used to investigate reaction rates including the measurement of mass, gas volumes and tie
What is the Boltzmann distribution and list key features
-It is used to represent the energy of the particles in a sample of a gas at a given temperature
-The line starts at the origin, showing that o particles have zero energy
-The total area under the distribution curve is equal to the total number of gas particles
How do temperature changes effect the rate of the reaction
It increases the rate of reaction because:
-It gives more particles sufficient energy to break their bonds
-More particles collide on the right orientation (the most reactive parts) and react
What is dynamic equilibrium
a dynamic equilibrium exists in a closed system when the rate if the forward reaction is equal to the rate of the reverse reaction and the concentration of reactants and products do not change
How do catalysts change effect of the rate of the reaction
A catalyst increases the rate of both the forward and reverse equations in an equilibrium by the same amount resulting in an unchanged position of equilibrium
The techniques and procedures used to investigate changes to the position of equilibrium for changes to the position of equilibrium for changes in concentration
Groups
Elements in the same group that have the same number of electrons in their outer shell and have similar physical and chemical properties
Periods
(horizontal rows) have their highest energy electrons in the same shell
Periodicity
A repeating trend in properties across each period
Ionisation energy
refers to energy needed to remove electrons
Atomic radius effect on nuclear attraction
The greater the atomic radius the smaller the attraction
Nuclear charge effect on nuclear attraction
The greater the nuclear charge the greater the attraction
Electron shielding effect on nuclear attraction
The greater the no. of shells, the greater the shielding, the less attraction
First ionisation energy
The energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ ions
Second ionisation energy
The energy required to remove one electron from each ion in one mole of gaseous 1+ ions to form one mole of gaseous 2+ ions.
Trend of 1st ionisation energy down a group
Ionisation energy decrease down a group because the atomic radius increases, shielding increases and nuclear attraction decreases
Giant covalent structures
Billions of atoms held together by a network of strong covalent bonds (boron, carbon and silicon)
About Graphite
Forms a giant planar structure with many layers held weakly together
About diamond and silicon
Form a giant 3d structure with atoms bonded in a tetrahedral arrangement. Not conductive as a;; 4 outer shell electrons are involved in covalent bonding
About group 2 metals
S out shell. Low densities. Reactivity increases as you go down the group. Second and first ionisation energies decrease down the group (as radius increases , shielding increases and attraction decreases)
Group 2 metals are ______ agents because...?
String reducing agents because they are a substance that adds electrons to another species and loses electrons
Redox reaction of group 2 metal with oxygen
2X(s) + O2 (g) ----> 2XO (s)
Redox reaction of group 2 metal with water
X(s) + 2H2O (l) ----> X(OH)2(s) + H2(g)
Redox reaction of group 2 metal with dilute acids
Metal + acid ----> salt + hydrogen
Mg + 2HCl -----> MgCl2 (aq) + H2 (g)
Group 2 oxides reaction with water
X(OH) + 2H2O (l) ----> X(OH)2
Usage of calcium hydroxide
Used to neutralise acids in soil
Ca(OH)2(s) + 2HCl (aq) -----> CaCl2 + 2H2O
Usage of magnesium hydroxide and calcium carbonate
Used to neutralise acids in stomach that cause indigestion
Mg(OH)2 (s) + 2HCl -----> MgCl2 (aq) + 2H2O (l)
CaCO3(s) + 2HCl(aq) -----> CaCl + H2O(s) + CO2(g)
About halogens
Exist as diatomic molecules. Form simple molecular lattices. Down the group London forces increase. Down a group number of shells increase and attraction decreases
Halogens are strong _____ agents
oxidising agents
Redox reactions between halogens and aqueous halides
Cl2 reacts with Br- and I-
Br2 only reacts with I-
I2 does not react
Cl2 (aq)+ 2BR (aq)----> 2Cl- (aq) + Br (aq)
Disproportionation of chlorine in water and the usage and disadvantages of usage
Cl2 + H2O -----> HCLO + HCL
Used in water treatment to kill bacteria. Chlorine is toxic and can react with hydrocarbons to form chlorinated hydrocarbons which are carcinogens.
Test for carbonate
Add dilute nitric acid to the sample. Effervescence passed through lime water which should turn cloudy if positive
CaCO3 (s) + HNO3 (aq) -----> Ca(NO3)2 (s) + CO2 (aq) + H2O (l)
Test for sulfate
Add aqueous BaCl2 or Ba(NO3)2 which should show a white precipitate
Test for halogens
Add aqueous silver nitrate Cl= white Br=Cream I= Yellow
Test for ammonia
Add aqueous NH^4+to the solution, warm then damp litmus paper from blue to red
Ammonia test for halogens
Chlorine dissolves in both dilute and concentrated ammonia
Bromine only dissolves in concentrated ammonia
Iodine does not dissolve in ammonia at all
What is metallic bonding
Metals form cations arranged in a regular lattice structure, the outer shell electrons are delocalised into a sea of electrons
Exothermic profile diagram
Endothermic profile diagram
What are standard conditions
100 kPa, 298K and 1 moldm^-3
Standard enthalpy change of reaction
The enthalpy change that accompanies a reaction in the molar quantities shown in a chemical equation under standard conditions, all reactants and products being in their standard states.
Standard enthalpy change of formation
The enthalpy change that takes place when one mole of a compound is formed from its constituent elements in their standard states under standard conditions.
Standard enthalpy change of combustion
The enthalpy change that takes place when one mole of a substance reacts completely with oxygen under standard conditions, all reactants and products being in their standard states.
Standard enthalpy change of neutralisation
The enthalpy change when an acid and alkali react together to form one mole of water, under standard conditions
Example of enthalpy change of reaction
2Na (s) + Cl2 ----> 2NaCl (s)
Example of enthalpy of change of formation
2Al (s) + 1.5O2 (g) ----> Al2O3 (s)
Example of enthalpy change of neutralisation
H+ (aq) + OH- (aq) -----> H2O (l)
Example of enthalpy change of combustion
CH4 (g) + 2O^2 (g) ---> CO2 (g) + H2O (l)
Equation of heat energy change
q (KJ) =mc∆T
m=mass in g
c= specific heat capacity in Jg^-1K^-1
∆T= temperature change in K
Errors in energy change of a reaction experiment (alkali+acid in a polystyrene cup) and solutions
-Heat loss
Solutions:
-Add lid to the polystyrene cup
-Adding insulation around the polystyrene cup
Errors in an enthalpy change of combustion experiment
-Heat loss to the surroundings
-incomplete combustion of fuel
-Evaporation of the fuel from the wick
-non-standard conditions being used
solutions:
-adding a lid to the beaker
-using draft shields around the apparatus
About making and breaking bonds
Breaking bonds:
-It is endothermic (needs energy)
-when more energy is needed to break bonds than is released when new bonds are formed, the overall energy is endothermic. -∆H
Making bonds:
-It is exothermic (releases energy)
-When more energy is released in forming new bonds than is needed to break bonds, the overall energy change is exothermic +∆H
About activation energy
Energy required to break bonds. Reactions with small activation energies generally take place very rapidly. Large activation energies may present such a large energy barrier that a reaction may take place extremely slowly.
Average bond enthalpy definition
Is the mean amount of energy required to break one mole of a specified type of covalent bond in a gaseous molecule. A-B (g)---> A(g) + B (g)
What are the limitations of using average bond enthalpies
Because the average bond enthalpy is only an average the actual value of a particular molecule could be slightly different and so is not accurate
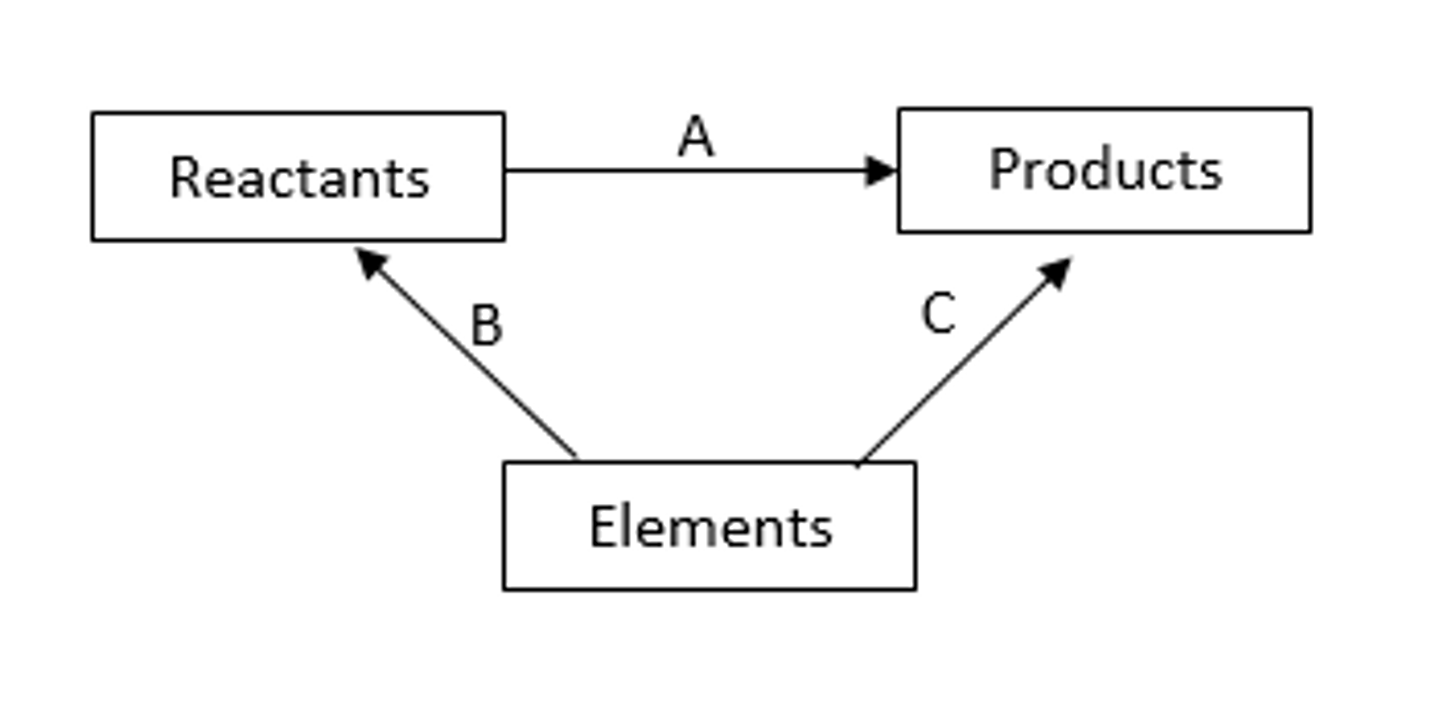
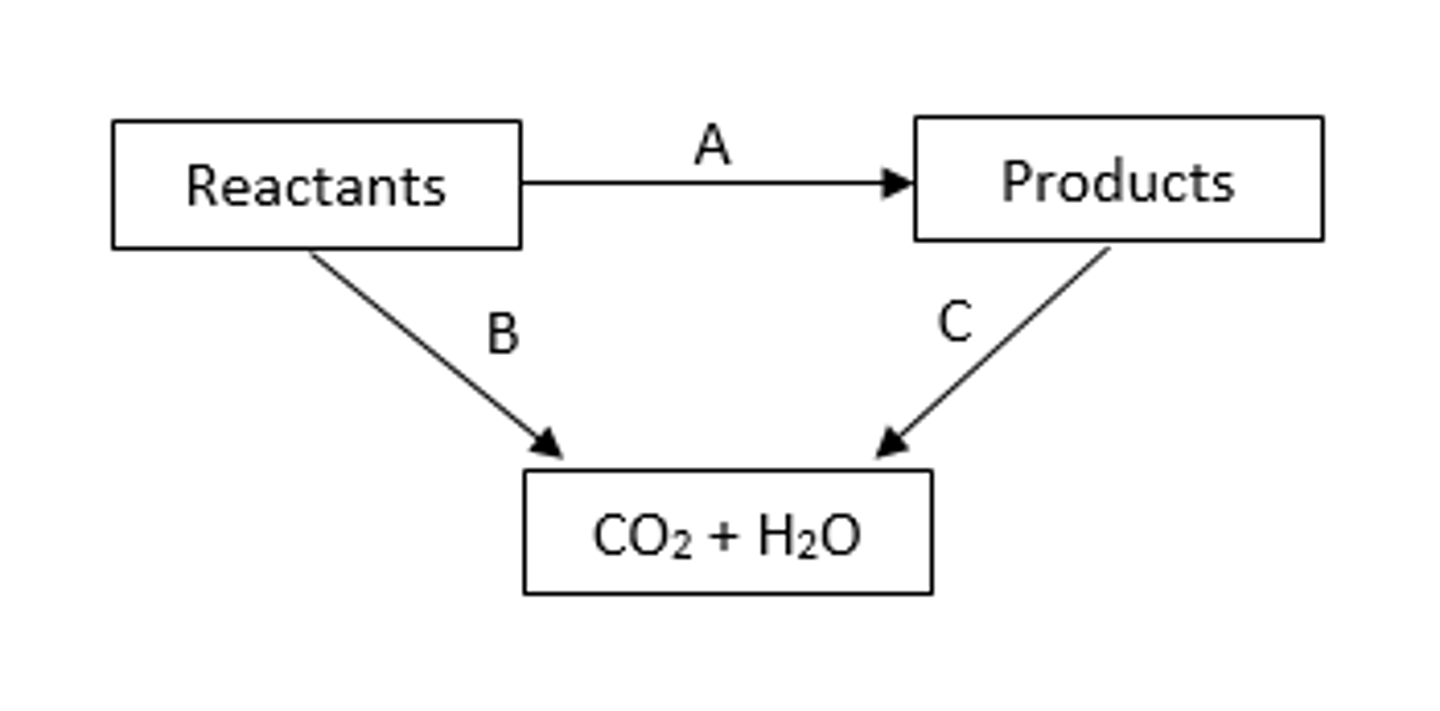
Hess' law definition
It states that if a reaction can take place by more than one route, and the starting and finishing conditions are the same, the total enthalpy change is the same for each route
Using Hess' law with enthalpy changes of formation diagram
Using Hess' law with enthalpy changes of combustion diagram
Bond enthalpies equation
∆rH=∑(bond enthalpies in reactants) -∑(bond enthalpies in products)
Two main points of the collision theory
-The colliding particles must have enough energy to break existing bonds
-The particles must be in the correct orientation so that the reactive parts of the particles collide
The lower the activation energy the _______ number of particles that can reaction and the ________ the reaction rate
larger, faster
Increasing the concentration has what effect on the rate of reaction?
Increase in concentration mean an increase in collision frequency which means an increase in rate of reaction
Increasing the pressure has what effect on the rate of reaction?
An increase in pressure means an increase in concentration which means an increase in successful collision which means an increase in rate of reaction
To find any rate at any time
What are the economical and environmental benefits of using catalysts
Many reactions used in the industry use energy but using catalysts lowers energy demands, which means less fossil fuels are burned as it lowers the temperature and pressure needed for a reaction to take place and also makes considerable cost savings
Effect of a higher temperature on Boltzmann distribution curve
At a higher temperature the peak is lower and shifts to the right this is because:
Peak shifts to the right- because average energy of particles increases
Peak lowers- because total number of particles remains the same
Catalysts effect on Boltzmann distribution curve
It does not change the distribution curve however a greater proportion of particles exceed the activation energy and it increases the rate of reaction