periodic trends
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
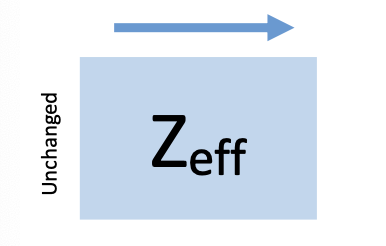
Zeff
pull between nucleus and valence e-
increase from left to right
2
New cards
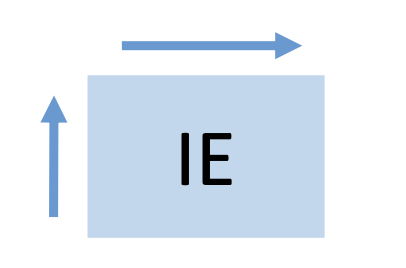
ionization energy
willingness to lose an electron
increase from left to right and down to up
3
New cards
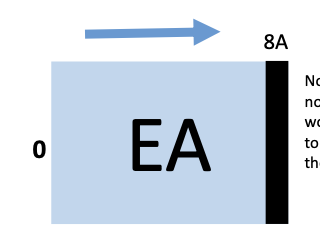
electron affinity
increase from left to right
4
New cards
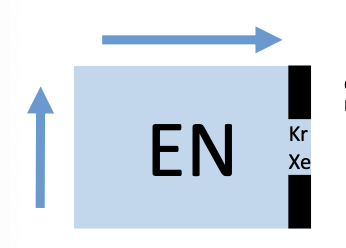
eletronegativity
increase from left to right
force the atom exerts on an electron in a bond
5
New cards

atomic size
increase from right to left and up to down