Red wine polyphenolic chemistry: mouthfeel, colour expression and stability
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
What are the two categories of grape and wine phenolics?
Non-flavonoid and flavonoid
may have one phenol ring or multiple phenol rings (polyphenol)
What are the non-flavonoid phenols?
hydroxycinnamic acids - drives colour in white wine - found in pulp/stems
benzoic acids
hydrolysable tannins - oak derived
stilbenes
What are the flavonoid phenols?
flavan-3-ols (monomeric and polymeric) - tannins! found in skins and seeds
catechin and epicatechin
procyanidins/proanthocyanidins
pigmented tannins
flavanols - found in skins - important one is quercetin (natural sunscreen in skins)
anthocyanins - colour - found in skins
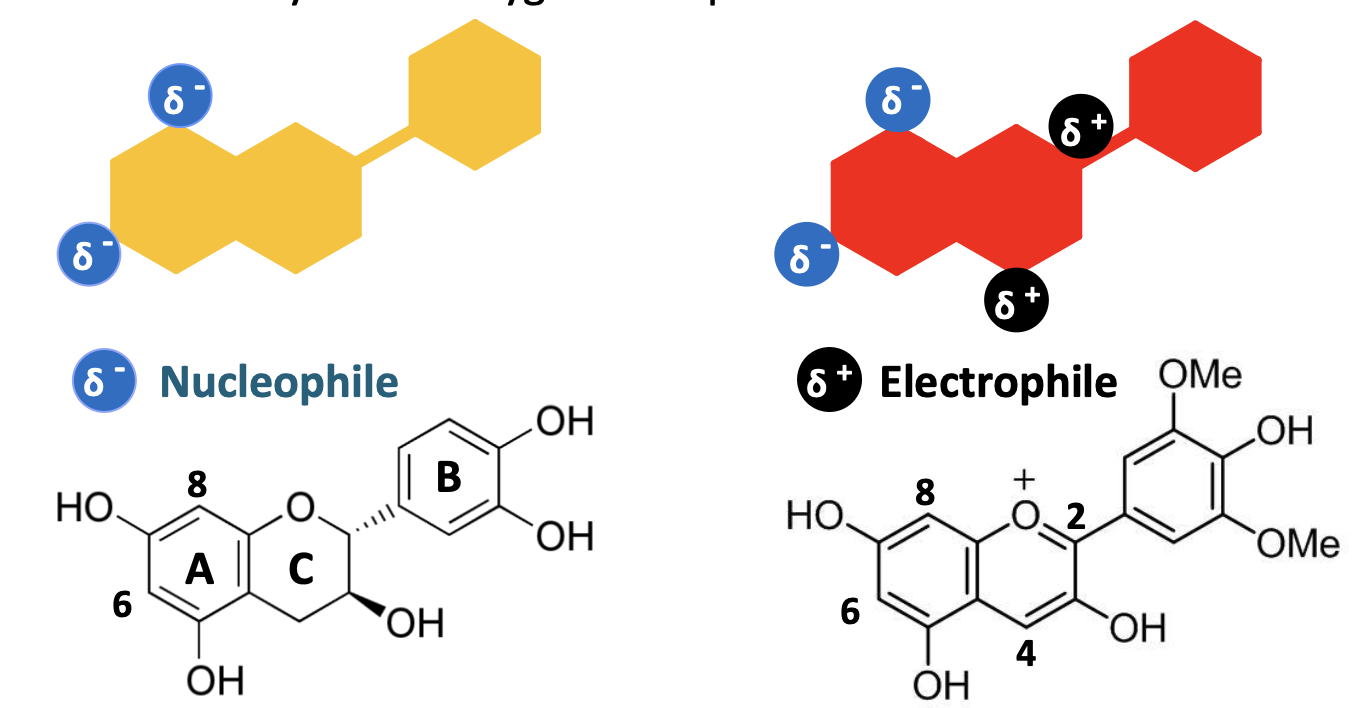
What occurs in the reactivity of flavonoids?
reactive due to electron density at various positions - high density regions = nucleophillic, will react with low density regions = electrophillic
electron density due to oxygenation pattern and double bonds
delta sign is used to indicate partial charge, so partial negative charge on the tannin (yellow) and partial positive charge on the anthocyanin (red)