elsaid - alkylating agents, platinum compounds, and antimetabolites
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
alkylating agents MOA
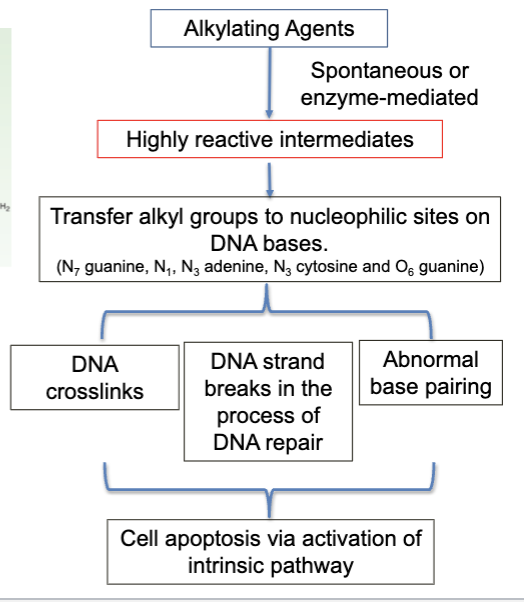
reacts with N7 guanine and forms alkylated base or a cross-link
alkylating agents:
nitrogen mustards
mechlorethamine
chorambucil
melphalan
cyclophosphamide
ifosfamide
alkylating agents:
nitosureas
carmustine
lomustine
alkylating agents:
alkylsulfonates
busulan
alkylating agents:
triazenes
procarbazine
dacarbazine
temozolomide
cyclophosphamide
alkylating agent
main organ-specific toxicity is hemorrhagic cystitis due to generation of Acrolein
Acrolein reacts with bladder tissue proteins and results in cell death and inflammation
ifosfamide
alkylating agent
generates acrolein and may cause hemorrhagic cystitis
generates chlordacetaldehyde
the generation of this metabolic byproduct causes renal tubular cell toxicity characteristic of ifosfamide
mesna (2-mercaptoethane sulfonate sodium)
cytoprotective agents
MOA:
detoxification of Acrolein via chemically reacting with the toxic metabolite
use:
to reduce risk of hemorrhagic cystitis associated with high-dose Ifosfamide or Cyclophosphamide
amifostine
cytoprotective agents
MOA:
a prodrug that is converted by membrane-bound AP
the active metabolite has a free -SH group
uses:
to reduce risk of radiation-induced xerostomia in patients with head and neck cancer
to reduce cumulative renal toxicity associated with Cisplatin in advanced ovarian cancer
platinum coordination complexes
cisplatin
carboplatin
oxaliplatin
platinum compounds MOA
formation of intra-strand DNA adducts or crosslinking DNA strands or DNA to protein
cisplatin MOA
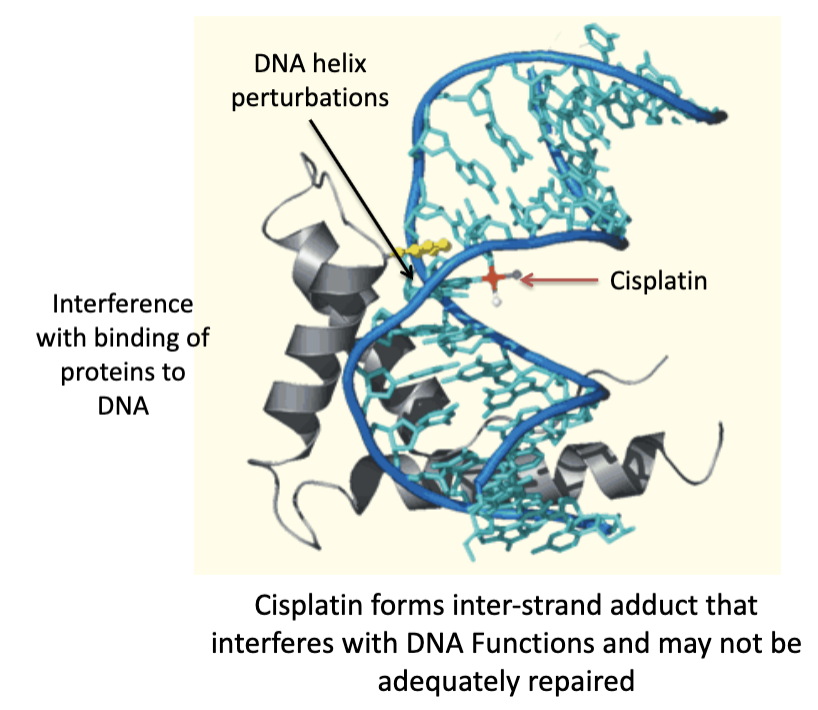
mechanisms of resistance towards Cisplatin
Cisplatin is taken up by specific transporters (copper transporter, organic cation transporters OCT)). Cisplatin is activated and forms DNA cross links (also called lesions).
reduction in expression of transporters responsible for uptake of Cisplatin
detoxification of reactive Cisplatin species by glutathione (GSH)
after formation of DNA crosslinks, enhanced nucleotide excision repair (NER) (a mechanism of DNA repair) causes removal of these lesions
alternatively, cancer cell can tolerate the formation of crosslinks and the DNA can be replicated as termed trans lesion synthesis (TLS)
the enzymes moving along the DNA strand jumps over the lesion area and continues its function
The formation of DNA crosslinks that are not adequately repaired triggers apoptosis. A mutation in p53 protein that results in loss of its function may reduce the probability that the cancer cell would undergo apoptosis and would result in clinical resistance
cisplatin nephrotoxicity
cisplatin-induced nephrotoxicity is a well-documented adverse effect
reduction of nephrotoxicity can be achieved with vigorous hydration and/or the use of amifostine
cisplatin causes nephrotoxicty due to necrosis of proximal tubular epithelial cells
proximal tubular epithelial cells have elevated levels of expression of organic cation transporter resulting in enhanced Cisplatin uptake and intracellular accumulation
mechanisms of proximal tubular necrosis and apoptosis by Cisplatin
cisplatin is uptaken by proximal tubular cells via copper and OCT transporters
cisplatin is activated and forms DNA crosslinks
the detoxifying capacity of proximal tubular cells is limited
the p53 protein is activated resulting in activating intrinsic apoptosis
cell damage occurs which reuslts in an irreversible loss in renal function
carboplatin
shares similar clinical efficacy spectrum to that of Cisplatin
Carboplatin is advantageous in the lack of renal toxicity evident with Cisplatin
Cisplatin is still being used b/c it’s superior in some cancers & carboplatin has ↑ risk of bone marrow suppression
antimetabolites
a class of drugs that by virtue of its structural similarity to purine or pyrimidine bases or important cofactors (e.g. folic acid)
inhibit the de-novo synthesis and DNA strand synthesis
de-novo synthesis is mandatory for cancer cells to meet the growth and cell division demands
these drugs are classified as S-phase specific agents
the cells are most susceptible to the cytotoxic effect of these agents as they enter the S-phase
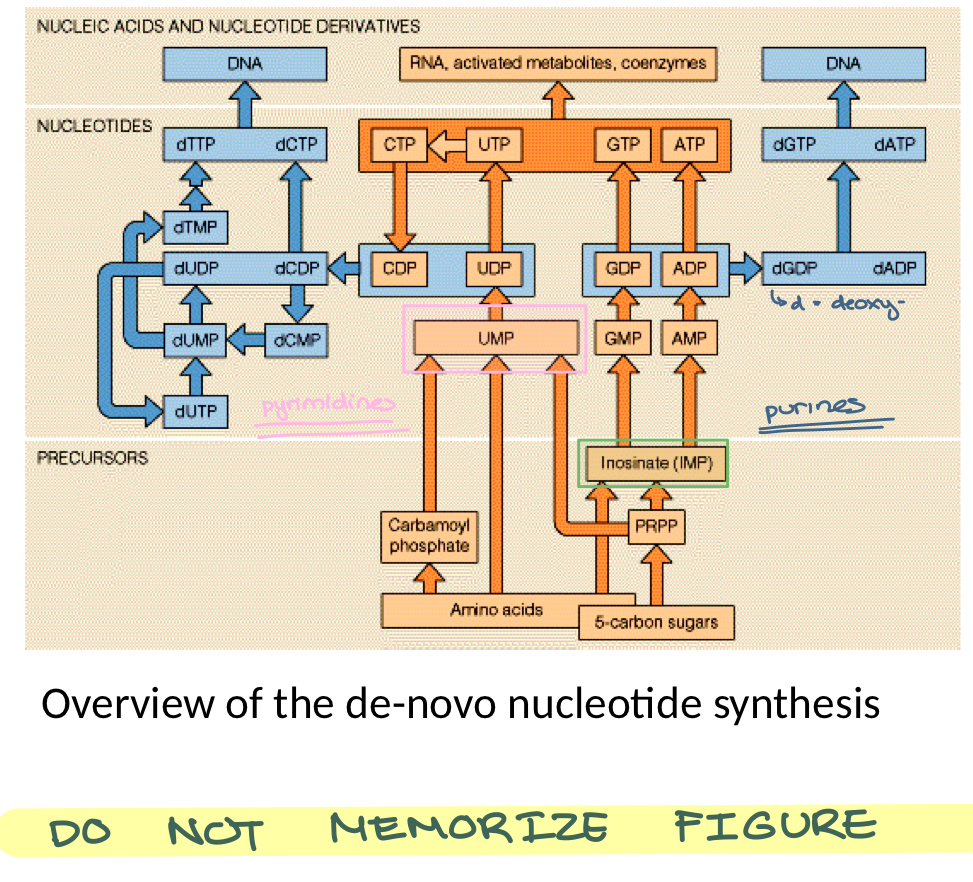
de-novo nucleotide synthesis
precursors stage
inosine monophosphate (IMP) is converted to GMP and AMP (purine bases)
uridine monophosphate (UMP) is converted to Cytosine diphosphate (CDP)
these are ribonucleotides (orange colored) that are converted to deoxyribonucleotides (blue) for incorporation into the DNA
deoxythymidine monophosphate (dTMP) is generated from deoxyuridine monophosphate (dUMP)
antifolates:
agents
methotrexate
pralatrexate
pemetrexed
antifolates MOA
these drugs are structurally similar to folic acid (an important enzyme cofactor)
they compete with folic acid on binding to enzymes that require folic acid
folic acid is essential in the de-novo synthesis of purine bases and in synthesis of dTMP
antifolates:
main sites of action
inhibition of conversion of dUMP to dTMP
no pyrimidines
inhibition of generation of inosine monophosphate (IMP)
no GMP & AMP —> no purines
pemetrexed
more potent form of MTX bc it binds with higher affinity
pemetrexed MOA
inhibition of dihydrofolate reductase (DHFR)
the enzyme responsible for regenerating tetrahydrofolate (THF) from dihydrofolate (DHF), allowing for the continued synthesis of purines and thymidylate
inhibition of thymidylate synthase (TS)
the enzyme responsible for conversion of dUMP to dTMP for DNA synthesis
inhibition of GARFT
the enzyme responsible for generation of AMP and GMP (purine nucleotides for DNA and RNA synthesis)
methotrexate:
uptake into cells
mediated by folic acid transporter
once in cytosol, MTX is polyglutamated by folate polyglutamats synthase (FPGS)
folate polyglutamate hydrolase (FPGH) can hydrolyze MTX-PG to MTX (which can diffuse back outside the cell)
MTX is trapped in the cancer cell by being polyglutamated
methotrexate:
targets
DHFR inhibition
thymidylate synthase
enzymes responsible for IMP biosynthesis
MTX-polyglutamate
the inhibitor of target enzymes resulting in inhibition of dTMP, AMP and GMP biosynthesis
Leucovorin
cytoprotectant
folinic acid (reduced form of folic acid —> tetrahydrofolate)
used as a chemoprotectant with antifolates to reduce the cytotoxic effect of antifolates on normal tissues
purine analogs:
inhibitors of AMP and GMP biosynthesis
mercaptopurine (6-MP)
inhibits AMP and GMP biosynthesis
thioguanine (6-TG)
inhibits GMP biosynthesis
6-MP and 6-TG:
toxicity
bone marrow suppression
6-MP and 6-TG:
activation
hypoxanthine guanine phosphoribosyl transferase (HGRPT)
6-MP —> T-IMP —> inhibits the synthesis of AMP and GMP
6-TG —> 6-thioGMP —> inhibits the synthesis of GMP
6-MP and 6-TG:
metabolism
6-MP:
metabolized by xanthine oxidase (XO) —> 6-thiouric acid
6-TG:
metabolized by thiopurine methyltransferase (TPMT) —> inactive metabolites
purine analogs:
inhibitors of DNA strand sytnehsis
Cladribine and Fludarabine
Cladribine and Fludarabine MOA
Cladribine and Fludarabine are converted to the triphospahte form and are incorporated into the nascent (developing) DNA strand
once incorporated, DNA strand elongation is terminated and cell undergo apoptosis
pyrimidine analogs:
inhibitors of dTMP biosynthesis
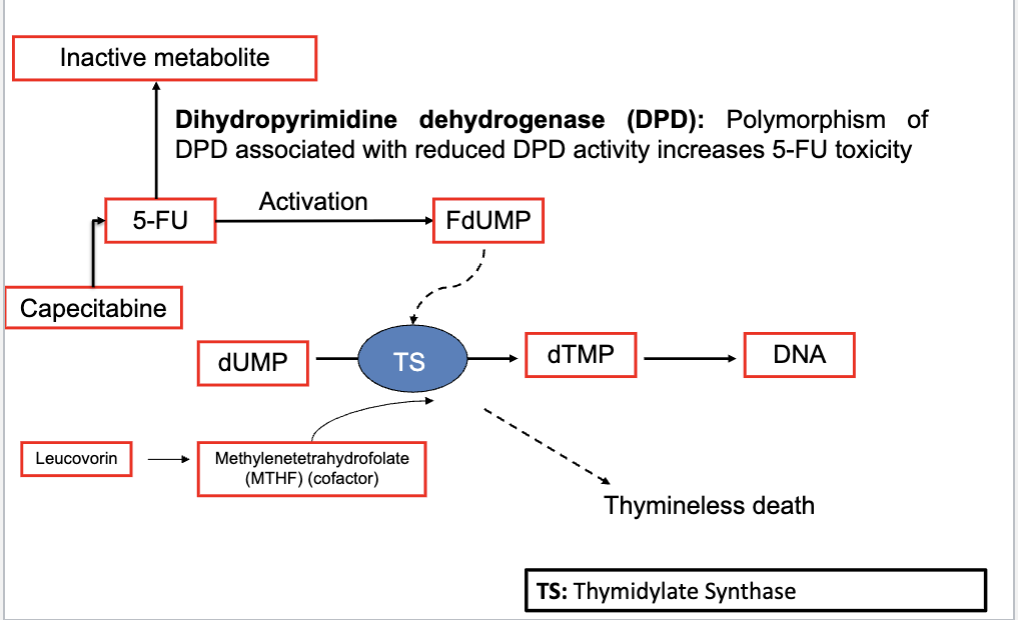
5-fluorouracil (5-FU) and capecitabine (prodrug)
MOA: inhibition of dTMP biosyntehsis
5-FU is administered with Leucovorin (potentiates the cytotoxic effect of 5-FU)
capecitabine and 5-FU metabolism and activation
pyrimidine analogs:
inhibitors of DNA strand synthesis
Cytarabine (Ara-C) and Gemcitabine are structural analogs to cytidine
Ara-C and gemcitabine are metabolized to triphosphate form which are incorporated in DNA
this results in chain termination and apoptosis
hydroxyurea
inhibition of ribonucleotide reductase that catalyzes the conversion of ribonucleotides to deoxyribonucleotides
an independent effect of hydroxyurea (unrelated to its inhibition of de-novo DNA synthesis) is its use in management of Sickle Cell Disease
hydroxyurea in SCD
hydroxyurea enhances fetal hemoglobin production (Hb F)
the Hb F- Hb S hybrids are less liekly to cause sickling of RBCs and results in veno-occlusion