nucleotides
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
compare ribonucleotides to deoxyribonucleotides
carbon 2 of ribonucleotides has OH, deoxy has just H
ribonucleotide has phosphate attached on C5, deoxy on C3
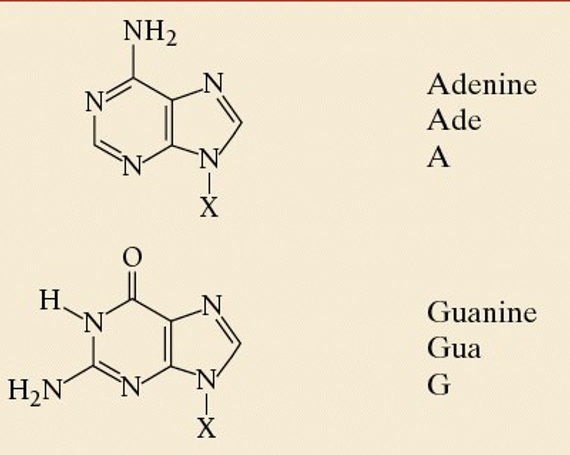
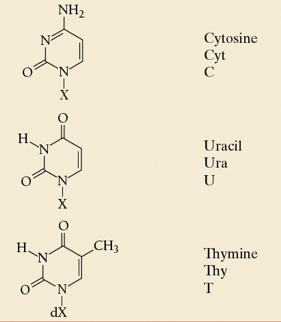
compare purines to pyrimidines
PURAG- purine is A and G, pyrimidines are C and T
purines have 2 ring shapes, pyrimidines have 1.
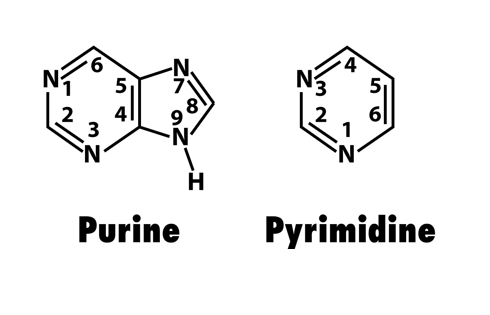
difference between the two purines
guanine is longer than adenine
difference between three pyrimidines
cytosine has only one C=O, uracil and thymine have 2, thymine has methyl group which uracil and cytosine lack
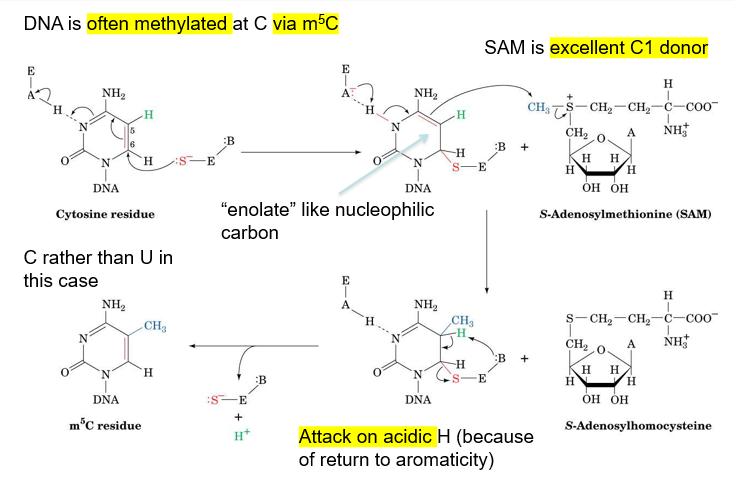
describe the methylation of cytosine
often occurs at cytosine, carbon 5
use enzyme with a cysteine residue, so that S- can attack carbon 6, making a C-S bond that makes C5 enolate-like and nucleophilic
C5 takes a methyl group from S-adenosylmethionine
base group from enzyme then deprotonates C5, breaking enzyme off cytosine and returning aromaticity to cytosine, making m5C
what number linkages are formed between DNA or RNA?
5’-3’
why is DNA said to have conformational freedom?
there is rotatable single bond character around sugar-phosphate linkages
what are syn and anti?
which conformations more common for the linkage between a purine base and a (deoxy)ribose?
syn and anti are rotational isomers, syn= same side, anti= facing away from each other
anti. this is because there are significantly more VDW clashes for syn than anti.
this means syn is higher in energy
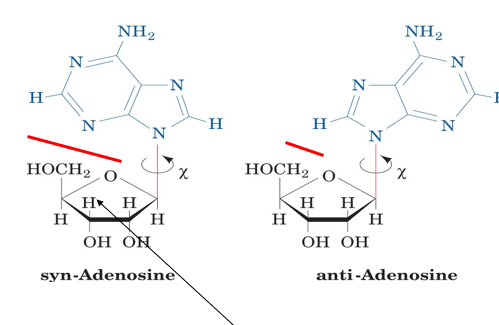
is syn or anti more common for a pyrimidine?
anti is more common
less steric hindrance for pyrimidine syn conformation than for purine, since pyrimidines are smaller
why is it better that sugar rings in DNA are puckered rather than planar?
puckering avoids VDW repulsion between substituents
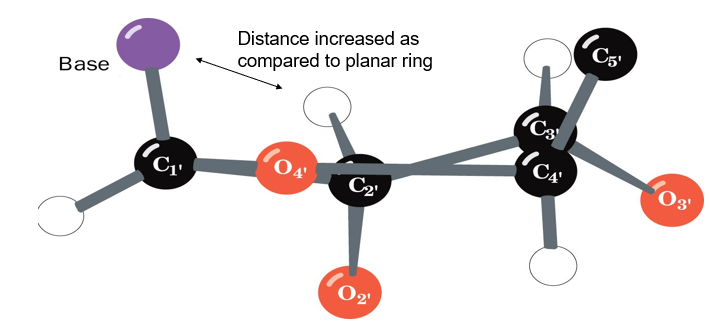
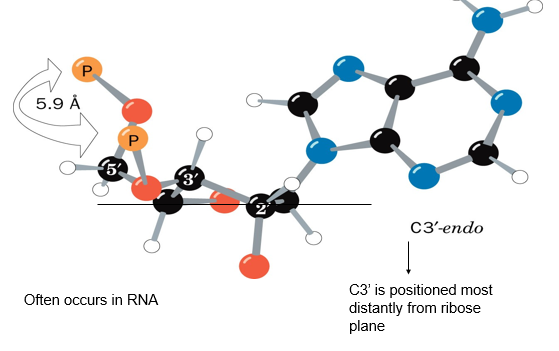
describe the puckering of ribose sugars in RNA
C3-endo’:
C3 of the ribose sugar is most distant from the ribose plane
5.9A between each of the two phosphatex that connects the nucleotide to others
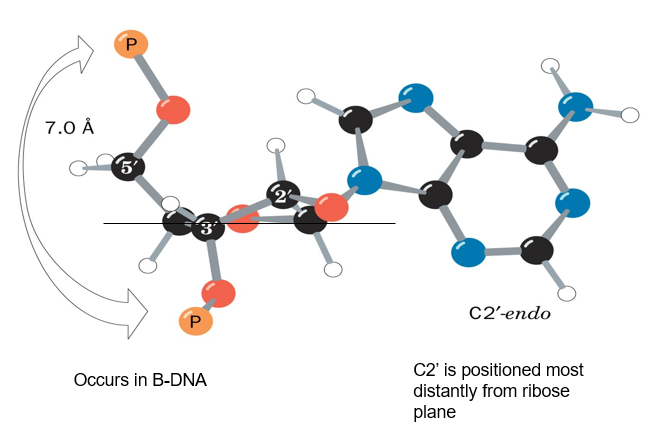
describe the puckering of deoxyribose sugars in B-DNA
C2-endo’:
C2 most distant from deoxyribose plane
7A between connecting phosphates
explain why deoxyribose and ribose are well-suited for their function
ribose has OH at C2, making it prone to cleavage. this means it’s not good for long-term storage, but can be used as a messenger molecule
deoxyribose doesnt have this OH at C2, making it more stable and useful for long-term storage
nucleotide reductases are one of the few examples of radical reactions. describe generally the 3 stages of radical reactions
initiation- homolytic fission. not heterolytic when it comes to nucleotide recutases
propagation- chain reactions where more radicals are being made from radicals
termination- radicals react to make normal product
describe the mechanism for the generation of deoxyribose from ribose via ribonucleotide reductases
enzyme has a stable radical, and propagation occurs, remove H from C3 of ribose and making ribose into a radical
enzyme then reduces a sulfur from one of its residues by giving the ribose C2 OH another hydrogen
presence of radical on C3 and protonation of C2 alcohol make C2 carbocation possible, done by removal of the H2O+
formation of a disulfide in the enzyme via reduction provides a hydrogen to give to the carbocation
radical propagation occurs, restoring the stable enzyme radical by giving a proton from the enzyme to the radical deoxyribose
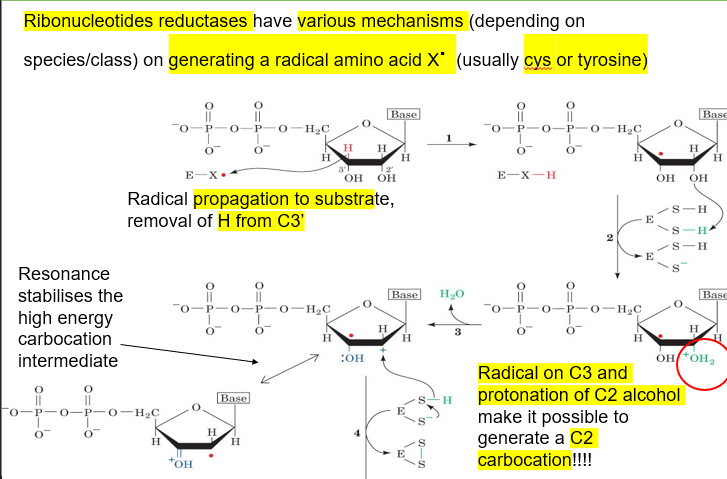
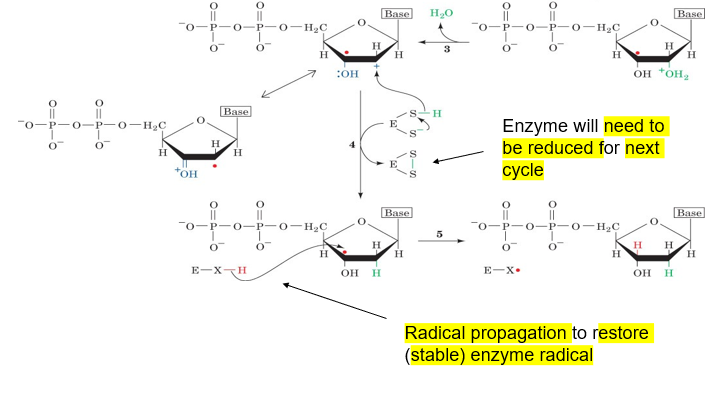
image showing the final step, formation of deoxyribose
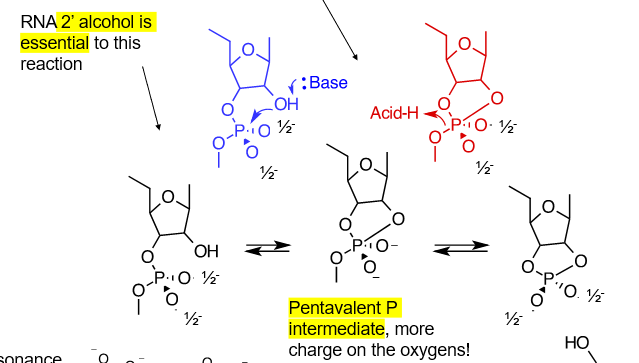
describe how RNAases work
acid/base catalysis
base deprotonates 2’ OH, while the oxygen bonds with the phosphate attached on the C3, and the phosphate then loses an oxygen to an acid
for this to occur, RNA must be unstructured, so that the C2 OH is close enough to the phosphate. trna and rrna is not as susceptible to RNAses/ hydrolysis
true or false: nucleotides’ only function is to store genetic material
false, they can also play catalytic roles, some RNAses are ribozymes, can cleave self
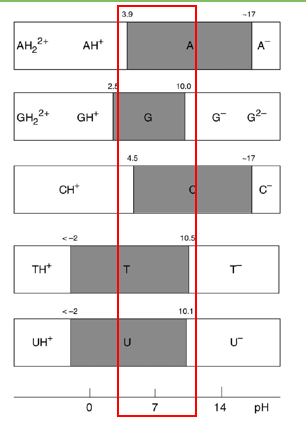
describe nucleotides’ propensity to perform acid-base chemistry
at pH7 they are all neutral and will not perform this chemistry
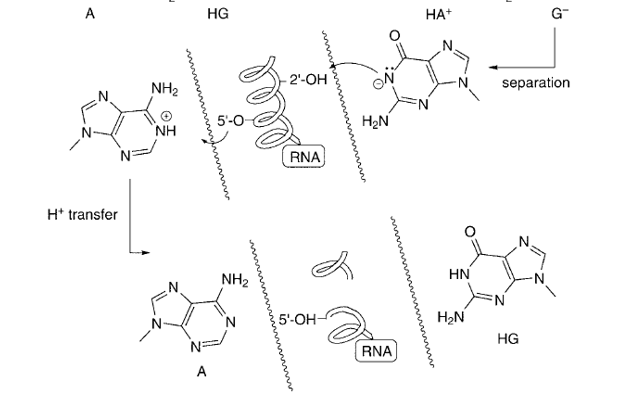
briefly describe how ribozyme catalysis works
faulty base pairs like A-G can be cleaved once proton transfer occurs.
proton transfer is between deprotonated guanine which takes proton from OH of ribose, which is recovered by taking a proton off protonated adenine
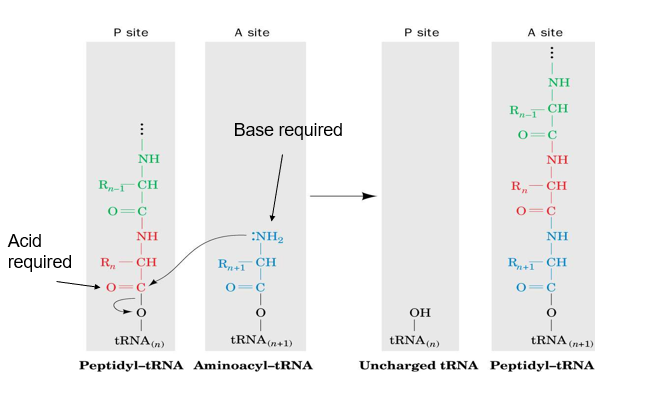
describe the catalytic site of a ribosome
the active site is made entirely of RNA, not protein. thus the ribosome is a ribozyme
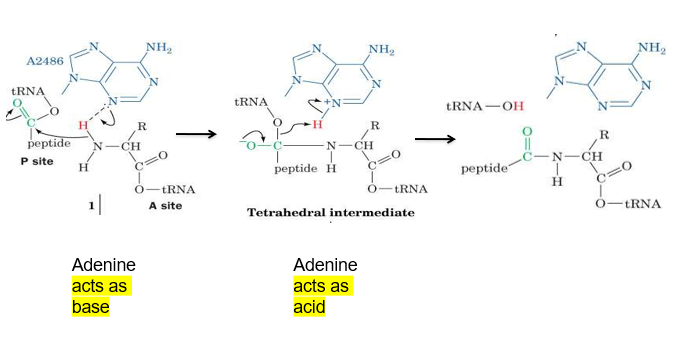
describe how adenine is proposed to allow the formation of a peptide bond in ribosome
adenine can act as a base first and deprotonate the amine group of one amino acid, allowing it to form a bond with the carbonyl group of another amino acid (attached to tRNA)
then the carbonyl group is reformed (from negative O), and the O binding tRNA to the amino acid breaks away from tRNA as it regains its H from protonated adenine, making carboxyl group