A-Level Chemistry Unit 1 Section 3
0.0(0)
Card Sorting
1/39
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
1
New cards
ions are formed
when electrons are transferred from one atom to another
2
New cards
compound ions
group of atoms with overall charge
3
New cards
ammonium
NH4 -
4
New cards
nitrate
NO3 -
5
New cards
hydroxide
OH -
6
New cards
carbonate
CO3 2-
7
New cards
covalent bonding
two or more atoms sharing electrons
8
New cards
single covalent bond
one shared pair of electrons
9
New cards
name the 2 giant covalent structures
graphite and diamond
10
New cards
low
simple covalent compounds have ____ melting points
11
New cards
high
giant covalent structures have ____ melting points
12
New cards
dative covalent bonds
both shared electrons come from the same atom
13
New cards
lone pairs
a pair of unshared electrons
14
New cards
charge cloud
an area where there is a chance of finding an electron
15
New cards
false
T/F bonding pairs repel more than lone pairs
16
New cards
a wedge means
the bond is pointing towards you
17
New cards
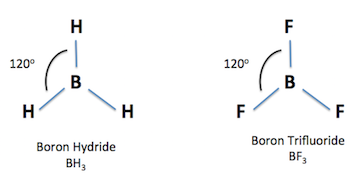
3 bonding pairs
trigonal planar bond angle 120°
18
New cards
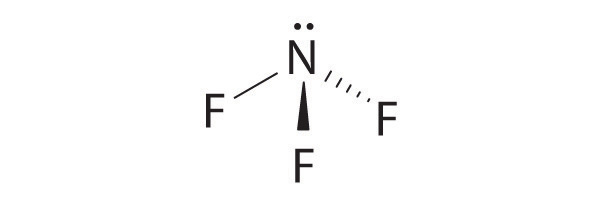
3 bonding pairs 1 lone pair
trigonal pyramidal bond angle 107°
19
New cards
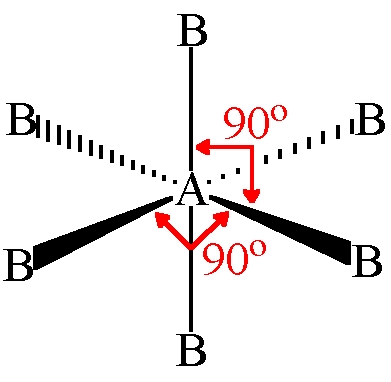
6 bonding pairs
octahedral bond angle 90°
20
New cards
electronegativity
an atoms ability to attract the bonding pair of electrons
21
New cards
polar bonds occur when
there is a difference in electronegativity between the bonding atoms
22
New cards
a molecule has a permanent dipole when
the charge is distributed unevenly over the whole molecule
23
New cards
polar molecules
molecules with a permanent dipole
24
New cards
3 types of intermolecular forces
van der waals
permanent dipole-dipole forces
hydrogen bonds
permanent dipole-dipole forces
hydrogen bonds
25
New cards
van der waals forces
between temporary dipoles. keep changing but present in all atoms
26
New cards
easy
van der waals forces are ____ to overcome
27
New cards
permanent dipole-dipole forces
in substances with permanent dipoles between δ- and δ+ charges on neighbouring molecules
28
New cards
hydrogen bonding occurs when hydrogen is bonded to
fluorine, nitrogen or oxygen
29
New cards
hydrogen bonding
hydrogen forms weak bonds with lone pairs from other atoms
30
New cards
hydrogen bonding gives molecules
uncharacteristically high melting/boiling points
31
New cards
metal elements structure
giant metallic lattice
32
New cards
metallic bonding
electrostatic attraction between positive metal ions and delocalised negative electrons
33
New cards
metal elements have high melting points due to
strong electrostatic forces of attraction between positive ions and negative electrons
34
New cards
good
metals are _____ thermal and electrical conductors
35
New cards
the melting and boiling point of a substance is determined by
the strength of attraction between its particles
36
New cards
polarity
solubility depends on
37
New cards
ionically bonded compounds have
high melting/boiling points and only conduct electricity when molten
38
New cards
simple covalent compounds have
low melting/boiling points and do not conduct electricity
39
New cards
giant covalent structures have
high melting/boiling points and do not conduct electricity
40
New cards
metallic structures have
high melting/boiling points and conduct electricity