C2 - Elements, compounds and mixtures
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Explain what is meant by the purity of a substance, distinguishing between the scientific and everyday use of the term ‘pure’.
In everyday use, the word ’pure’ means clean or natural. In scientific use, a substance is pure if it is completely made up of a single element or compound.
Use melting point data to distinguish pure from impure substances.
Every pure substance has a specific boiling point, so if the boiling/melting point is too low, the substance is impure.
Calculate relative formula masses of species separately and in a balanced chemical equation
Relative formula mass is the sum of all relative atomic masses in a formula.
Relative atomic mass is the average mass of an atom of an element in comparison to 1/12th of a carbon atom, which has a mass of 12.
Relative molecular mass is the sum of all relative atomic masses in a molecule.
Deduce the empirical formula of a compound from the relative numbers of atoms present or from a model or diagram and vice versa.
To deduce the empirical formula, divide by the highest common factor.
To find the molecular formula, divide the Mr of the molecular formula by the Mr of the empirical formula and multiply the number of atoms by that number.
Explain that many useful materials are formulations of mixtures.
Alloys are composed of a mixture that is at least 1 metal and another element. This is to enhance the properties of the base metal such as strength, hardness, or resistance to corrosion.
Explain the process of filtration
Filtration - Separating an insoluble solid from a liquid - pour the mixture through filter paper into a beaker.
Explain the process of crystallisation
Crystallisation - Separating a soluble solid from a liquid - evaporate around 2/3s of the solvent. Leave the dish out to naturally evaporate and crystals will form.
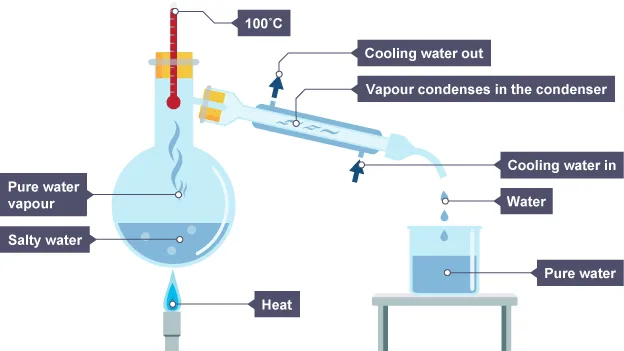
Explain the process of simple distillation
Separating 2 liquids with different boiling points.
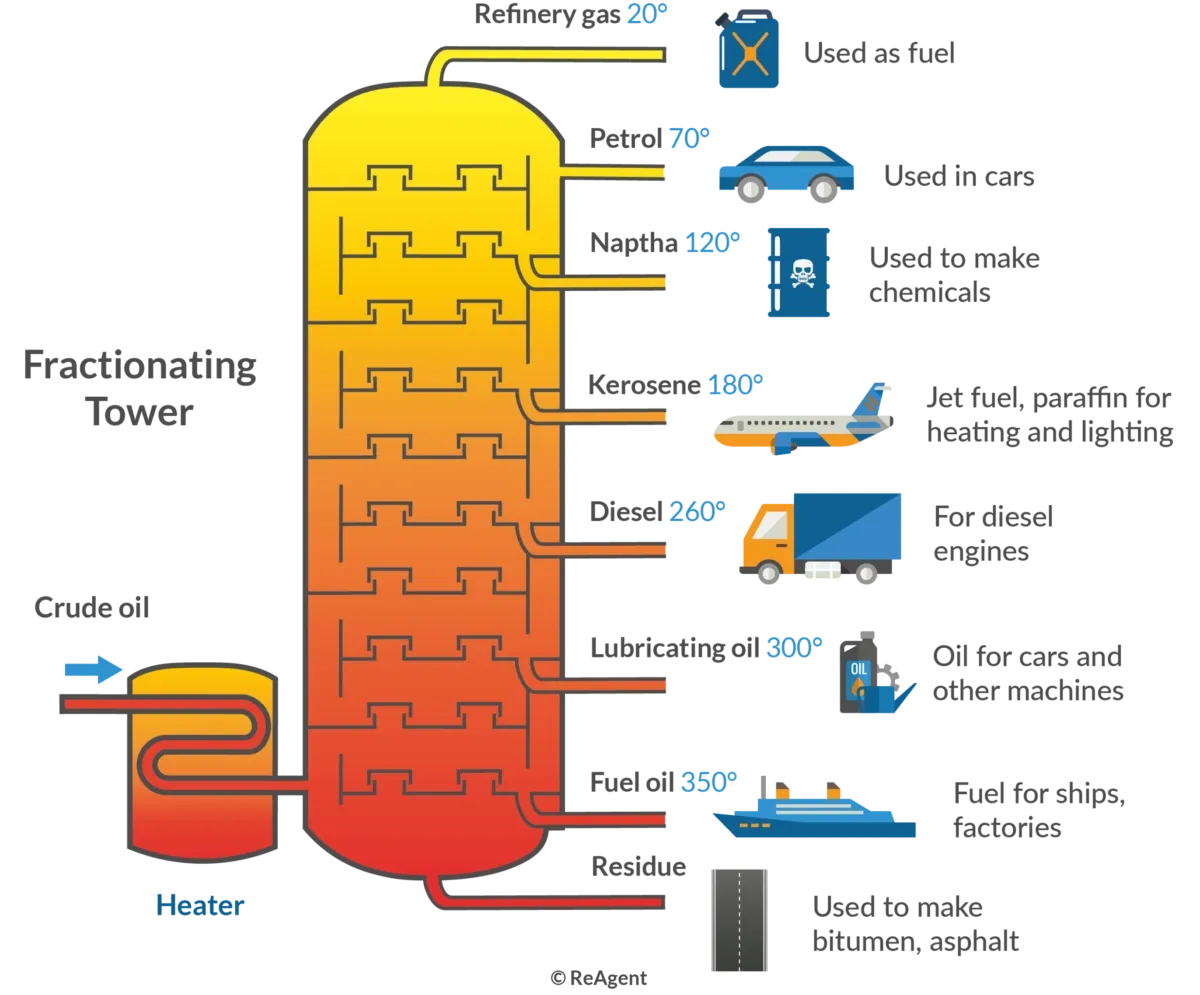
Explain the process of fractional distillation
Separating multiple liquids from a mixture.
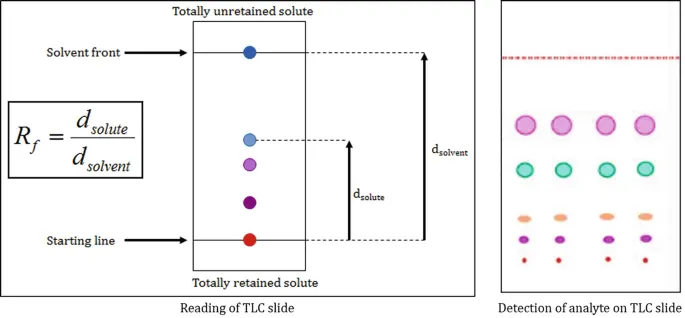
Describe the technique of paper and thin layer chromatography.
Thin layer chromatography, you must draw a line near the bottom of the plate using a pencil. Put a spot of mixture on this line. Put some solvent in a beaker, and dip the bottom of the plate in it. Place a cover on top of the beaker so solvent does not evaporate. The solvent will move up the plate, and carry the mixture as well. This will separate the chemicals in the mixture and allows us to identify the chemicals. Paper chromatography is the same but with chromatography paper instead of a plate.
The time taken for each phase depends on 2 things:
How soluble they are in the solvent.
How attracted they are to the stationary phase.
Molecules with a higher solubility and lower attraction will spend more time in the mobile phase so is carried further up.
Interpret chromatograms, including measuring Rf values.
Suggest suitable purification techniques given information about the substances involved.
If the substances involved are both liquids in a solution, you can use fractional or simple distillation to purify them.
If the substances involved are a soluble solid in a solution, you can use crystallisation.
If the substances involved are an insoluble solid in a solution, you can use filtration.
Suggest chromatographic methods for distinguishing pure from impure substances.
Thin layer chromatography, paper chromatography or gas chromatography.
Metal characteristics
Metals have high melting and boiling points and a high density as well. This allows them to be strong, but also malleable. They are good conductors of heat and electricity, and react with oxygen to form metal oxides.
Non-metal characteristics
Non-metals usually have low melting and boiling points, and when solid can be very weak and brittle. They have lower densities than metals and don’t conduct electricity, however there are exceptions.
Explain how the atomic structure of metals and non-metals relates to their position in the Periodic Table.
Metals are placed on the left hand side of the periodic table, and non-metals are placed on the right. This is because the outer electrons of each determine where they are in the table. They are separated by a zigzag line, however elements near that line may have properties of both - Metalloids.
Explain how the position of an element in the Periodic Table is related to the arrangement of electrons in its atoms and hence to its atomic number.
An element’s group number corresponds to the number of electrons it has in its outer shell. An element’s atomic number corresponds to the number of protons/electrons. The period an element belongs to is dependent on how many shells that element has.
Describe and compare the nature and arrangement of chemical bonds in Ionic compounds.
Ionic compounds have ionic bonding between ions and have a giant ionic lattice structure which form a densely packed regular lattice. These have very strong electrostatic forces of attraction between opposite ions.
Describe and compare the nature and arrangement of chemical bonds in simple molecules.
Covalent bonds between atoms by sharing pairs of electrons so both atoms have full outer shells. Covalent bonds are strong as they have strong electrostatic attraction between the nuclei and the electrons.
Describe and compare the nature and arrangement of chemical bonds in giant covalent structures.
Similar to giant ionic lattices except they have covalent bonds between atoms instead of electrostatic forces of attraction.
Describe and compare the nature and arrangement of chemical bonds in polymers.
Lots of small molecules join together to form polymers.
Describe and compare the nature and arrangement of chemical bonds in metals.
Metallic bonding is the electrostatic attraction between ions and electrons. The ions are surrounded by the electrons so the attraction acts in all directions.
Describe the limitations of particular representations and models.
Dot and cross diagrams do not show the structure of the compound.
Ball and stick models have incorrect scales since there are not gaps between ions and ions are different sizes.
3D representations only let you see the outer layer of the compound.
2D representations do not show bonding.
Explain how the reactions of elements are related to the arrangement of electrons in their atoms and hence to their atomic number.
Atomic number tells you the arrangement of electrons in an element. This tells you the number of valence electrons that the atom has. This tells how how that element tends to react, e.g. group 1 and 7 only have to lose/gain 1 electron so they are very reactive.
Explain in terms of atomic number how Mendeleev’s arrangement was refined into the modern Periodic Table.
Mendeleev put elements in order of atomic mass and arranged them with gaps to keep elements with similar properties together. The modern table has incorporated atomic number and adjusted it to fit those same patterns.
Explain why carbon is able to form so many different types of natural and synthetic compounds.
Carbon can form many different types of molecules because carbon atoms can form up to 4 covalent bonds, and bond easily with other carbon atoms to make chains and rings.
Explain the properties of diamond in terms of its structure and bonding.
Diamond is extremely hard as each carbon atom forms four covalent bonds in a very rigid covalent structure. These covalent bonds allow diamond to have a very high melting point. Diamond cannot conduct electricity as it has no free electrons or ions.
Explain the properties of graphite and graphene in terms of its structure and bonding.
Graphene and graphite - Each carbon atom forms 3 covalent bonds, creating sheets of atoms that are free to slide over each other. These layers are held weakly. Graphite has a high melting point as there are lots of strong covalent bonds . Graphite has lots of delocalised electrons as only 3 out of the 4 valence electrons are used in bonding, allowing it to conduct electricity. A single sheet of graphite is graphene, and is extremely strong and thin.
Explain the properties of fullerenes in terms of its structure and bonding.
Fullerenes are large molecules of carbon atoms shaped like spheres or tubes. The carbon atoms are arranged in rings and have delocalised electrons so they can conduct electricity. Melting and boiling points are high but lower than diamond since there are more intermolecular forces.
Explain why the structure of a compound affects the temperature at which change of state occurs.
Simple covalent structure has a low melting point as there are lots of weak intermolecular forces.
Giant covalent structure has a high melting point as there are lots of strong covalent bonds.
Metallic structures have high melting points as the metal ions are very strongly attracted to the sea of delocalised electrons.
Ionic structures have high melting points as there is strong electrostatic attraction between opposite ions.
Compare ‘nano’ dimensions to typical dimensions of atoms and molecules.
Nanoparticles are particles between 1 and 100nm across and contain roughly a few hundred atoms.
Describe the surface area to volume relationship for different-sized particles and describe how this affects properties.
As particles decrease in size, the size of their surface area increases in relation to their volume. This gives them different properties to larger particles as a much greater proportion of their atoms are available to interact with substances they come into contact with.
Describe how the properties of nanoparticulate materials are related to their uses.
Nanoparticles make good catalysts as they have a large SA:V ratio.
Nanoparticles are very small so can be used in sun creams, deodorants, Nanomedicine and lubricant coating.
Nanotubes conduct electricity so can be used in electrical circuits.
Nanoparticles are added to plastics in sports equipment to make the plastic stronger without adding as much weight.
Silver nanoparticles are added to polymer fibres in surgical masks and wound dressing giving the fibres antibacterial properties.
Explain the possible risks associated with some nanoparticulate materials.
The way some nanoparticles affect the body is not fully understood so its important that new nanoparticle products are tested thoroughly to minimise the risks.