Alkane, alkenes and alcohols
5.0(2)
5.0(2)
Card Sorting
1/25
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
1
New cards
what is the general formula for alkanes
CnH2n+2
2
New cards
what is the general formula for alkenes
CnH2n
3
New cards
what is a hydrocarbon
a compound containing only carbon and hydrogen atoms
4
New cards
definition of saturated compound
the compound has the maximum number of bonded atoms
5
New cards
definition of unsaturated compound
a compound does not have the maximum number of 4 bonded atoms
6
New cards
characteristics of alkanes
- single covalent bond
- saturated
- C-H + C-C single bond
- non-polar substances (very little attraction and no charged ends)
- will burn completely in sufficient oxygen
- saturated
- C-H + C-C single bond
- non-polar substances (very little attraction and no charged ends)
- will burn completely in sufficient oxygen
7
New cards
what is the suffix of alkanes
-ane
8
New cards
what is the suffix of alkenes
-ene
9
New cards
what is the test to differentiate between alkenes and alkanes
add bromine water to an alkane/alkene
10
New cards
what will happen when bromine water is added to an alkane
- it remains an orange-brown liquid
- it didn't decolourise
- it didn't decolourise
11
New cards
why does bromine water not decolourise with an alkane
because it is a saturated hydrocarbon
12
New cards
what will happen when bromine water is added to an alkene
- it turns clear
- decolourises the bromine water
- decolourises the bromine water
13
New cards
why does bromine water decolourise with alkenes
because alkenes are unsaturated hydrocarbons and are able to undergo an addition reaction for a new molecule to form
- double bond breaks and a spare electron covalently bonds with bromine
- double bond breaks and a spare electron covalently bonds with bromine
14
New cards
structural formula for bromine and water
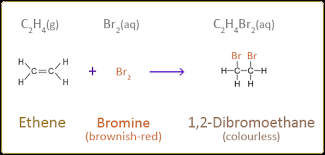
15
New cards
what does cyclohexane look like
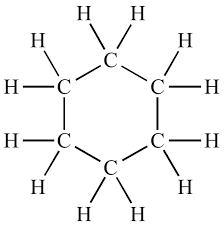
16
New cards
what does cyclohexene look like
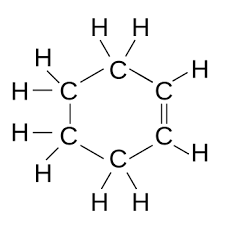
17
New cards
alkane and alkene solubility
it non-soluble in water because its a non-polar substance and negligible
18
New cards
characteristics of hydrocarbons
the longer the carbon chain the stronger the intermolecular forces
19
New cards
what is the general formula of alcohols
CnH2n+1OH
20
New cards
what state are alcohols at room temperature
liquids
21
New cards
why are alcohols easier to transport
its a liquid at room temperature
22
New cards
characteristics of alkenes
- double covalent bonds between two carbon atoms
- one carbon-carbon double bond per molecule
- unsaturated (double-bond can break)
- one carbon-carbon double bond per molecule
- unsaturated (double-bond can break)
23
New cards
why does methene not exist
since two carbon atoms are required to form a double bond (methane only has one)
24
New cards
alkanes summary
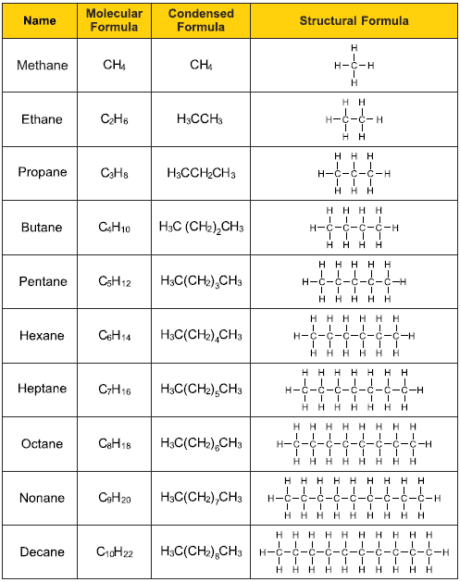
25
New cards
alkenes summary
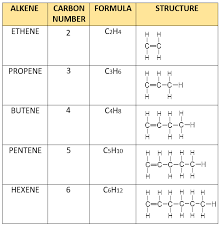
26
New cards
methanol and ethanol general formula
methanol - CH3OH
ethanol - C2H5OH
ethanol - C2H5OH