Phase Transformations - Exam 3
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
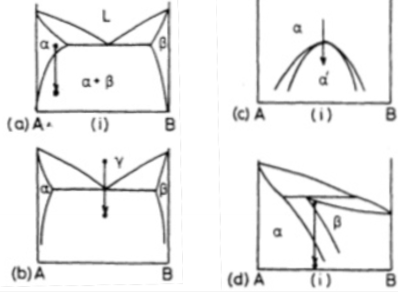
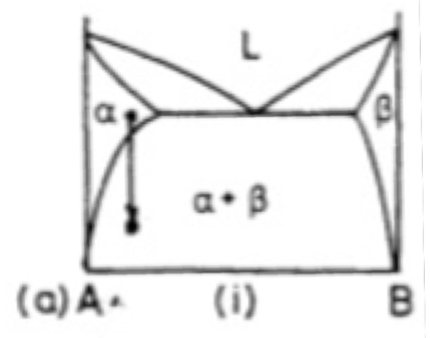
Which of the schematic phase diagrams indicates a precipitation reaction?
What are 5 types of diffusional transformations?
Precipitation reactions
Eutectoid transformations
Ordering reactions
Massive transformations
Allotropic transformations
Typically induced by a temperature change.
What is a precipitation reaction?
Second phase precipitates form from a supersaturated solid solution of the matrix phase
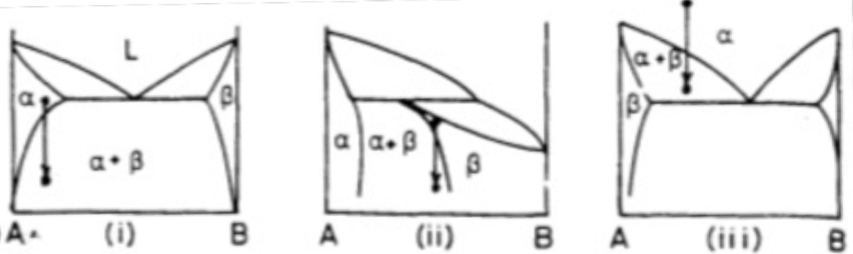
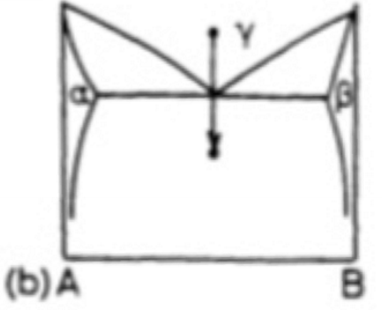
What is a eutectoid transformation?
A metastable solid phase is replaced by a more stable mixture of two other phases
What are ordering reactions?
Atoms rearrange to form a more stable ordered structure from a metastable disordered structure.
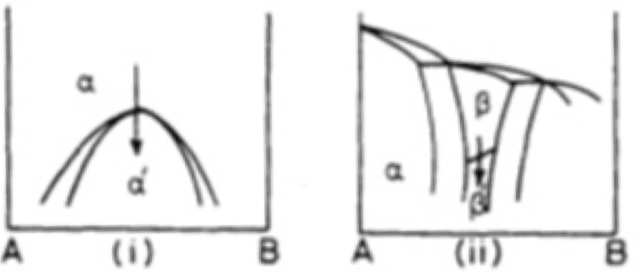
What are massive transformations?
Original phase decomposes into one or more new phases with same composition as the parent phase, but different crystal structure.
What are allotropic transformations?
Occur in single component systems when different crystal structure are stable over different temperature ranges.
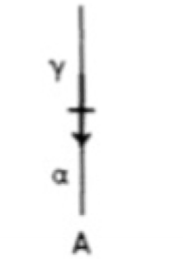
What is long-range diffusion and what types of transformations require this?
Diffusion that results in a large change in composition.
Precipitation and Eutectoid
How does an increase in the misfit strain energy affect the critical nucleus size?
Increases
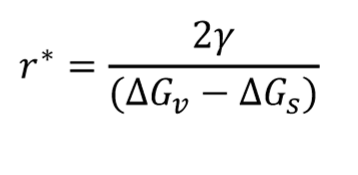
What is the effect of solute content on nucleation rate?
For alloys containing less solute, the critical undercooling will not be reached until lower absolute temperature where diffusion is slower.
What types of defects increases the free energy of the material?
All of them.
Specific examples could be given:
excess vacancies
dislocations
grain boundaries
inclusions
If the creation of a nucleus results in the destruction of a defect…what will occur?
Some energy will be released, thereby reducing or even removing the energy barrier.
What 6 defects act as heterogeneous nucleation sites? Put in order of the magnitude of increase in energy added to the system (1 lowest energy addition → 6 highest energy addition)
EDGSIF
Excess vacancies
Dislocations
Grain boundaries
Stacking faults
Inclusions
Free surfaces
How does the energy barrier for heterogeneous nucleation change with an increase ΔGd?
Decreases
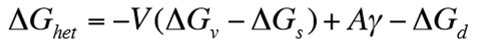
Since ΔGd is small for vacancies, what three parameters are needed in combination for nucleation to occur?
Low interfacial energy (i.e. fully coherent nuclei)
Small volume strain energy
High driving force
In what 3 ways can dislocations assist in nucleation?
Reduce ΔGs contribution to ΔG* by reducing total strain energy
Raise the composition nearer to that of precipitate by solute segregation
Reduce ΔGm (mobility term) by provided a diffusion pipe (“reduced activation energy for diffusion”)
Not effective at reducing interfacial energy contribution
Which of the following has the lowest energy barrier ΔG*? Why?
Homogeneous nucleation
Nucleation at vacancies
Nucleation on dislocations
Not enough info
Nucleation on dislocations - Dislocations have higher energy associated with them compared with vacancies.
Which of the following has the lowest energy barrier ΔG*?
Nucleation on grain boundaries
Nucleation on stacking faults
Nucleation on free surfaces
Nucleation on free surfaces

In the absence of strain energy effects, what 3 things are true of nuclei that form?
They have the smallest nucleation barrier
They have the smallest critical volume
They have the minimum total interfacial free energy
How does the precipitate growth rate change with an increase ΔX0?
Increases
Growth involves what three steps?
Volume diffusion of solute to the grain boundary
Diffusion of solute along the grain boundary with some attachment to precipitate
Diffusion along the α/β interfaces allowing accelerated thickening
What happens to the equilibirum composition of B in the α phase when the β phase shrinks?
It increases
What are the x and y axes of a TTT diagram?
Time, Temperature
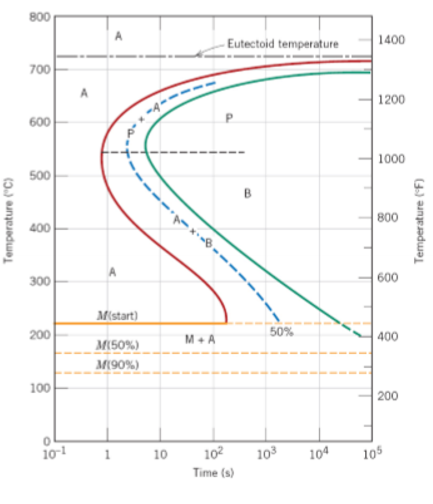
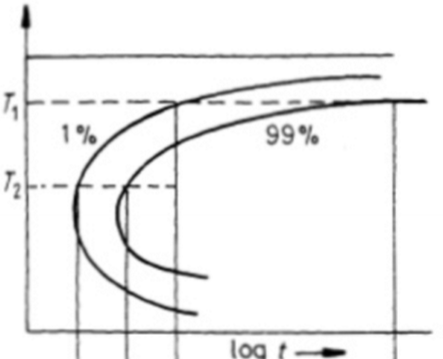
Why do TTT curves for civilian transformations generally have a ‘C’ shape?
At high temperatures there’s not that much driving force for transformation to occur so will tend to be slower. At low temperatures, the diffusion rate is low, so there isn’t a quick transformation.
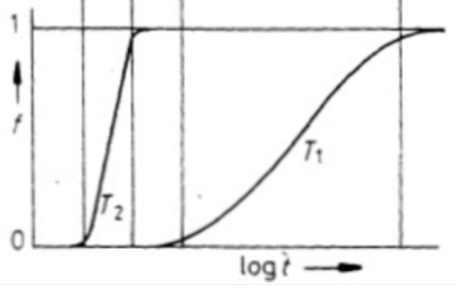
Why do the fraction transformed curves have a low slope at the beginning and end?
At the beginning, it will take some time for nucleation to occur once reaching the temperature. It will increase a smore nuclei are formed in the system. Towards the end of the transformation, B atoms will have to be pulled further from the matrix phase to form the precipitates. Also, impinging of adjacent transformed volumes would slow this process.
What are 5 factors that determine the fraction transformed?
Nucleation rate
Growth rate
Density and distribution of nucleation sites
Overlap of diffusion fields form adjacent transformed volumes
Impingement of adjacent transformed volumes
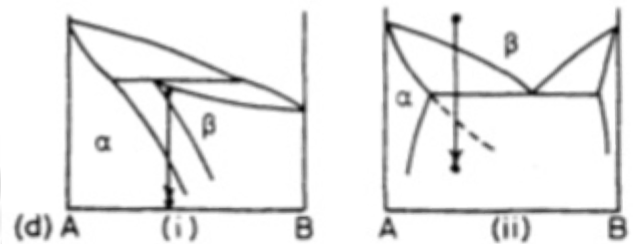
What are cellular transformations?
All parent phase is consumed by transformation product.
Which of the following is a type of cellular transformation?
Pearlite
Cellular precipitation
Massive transformations
Recrystallization
All of them.
Transformation of the type α → β or α → β + γ where all of the parent phase is consumed by the transformation product.
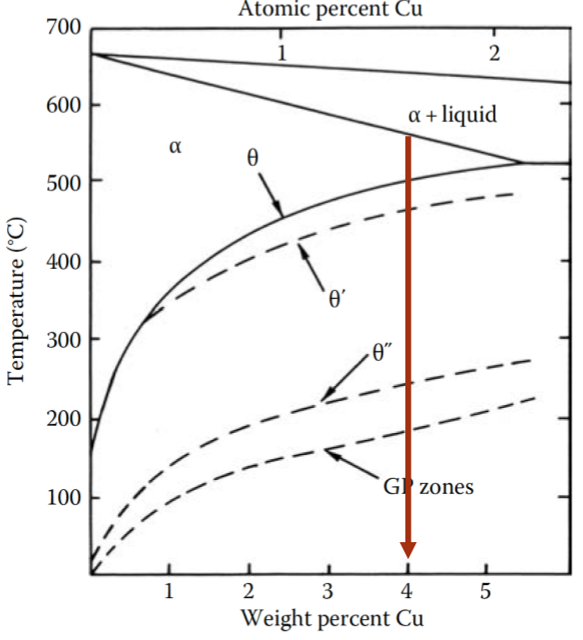
Consider the Al-Cu alloy, if we hold it at a temperature < 180°C, what are we doing?
Aging
What are GP zones?
“Guinier–Preston zones” are the first precipitate to nucleate. They minimize strain energy by forming disc-shapes perpendicular to elastically soft direction in FCC matrix
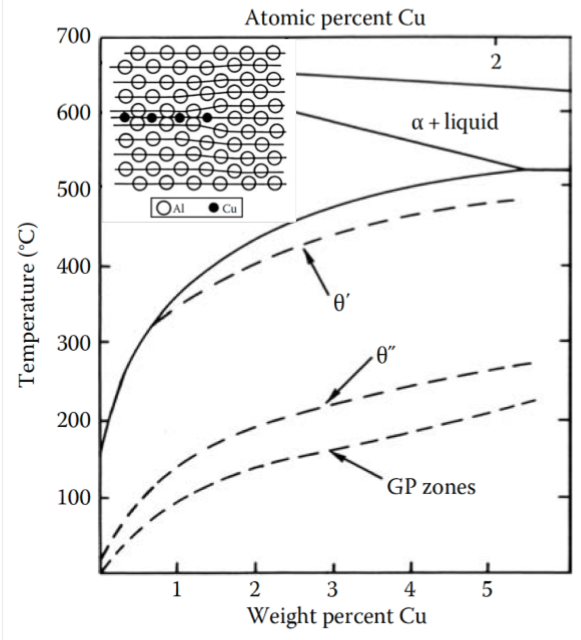
What precipitates after the formation of GP zones?
Transition phasesW
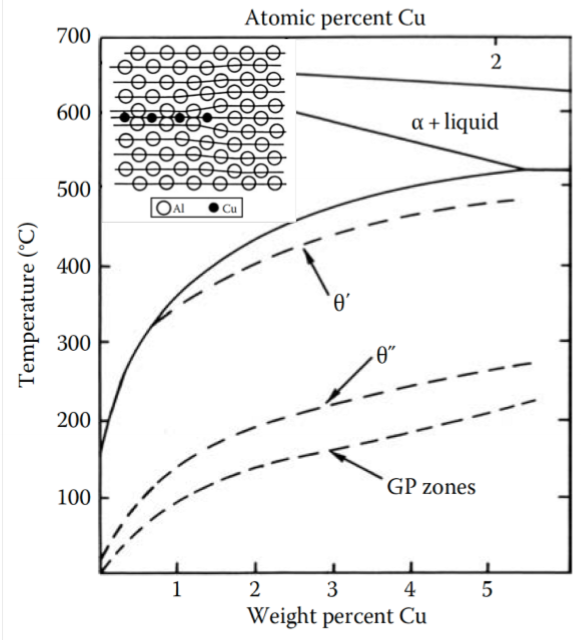
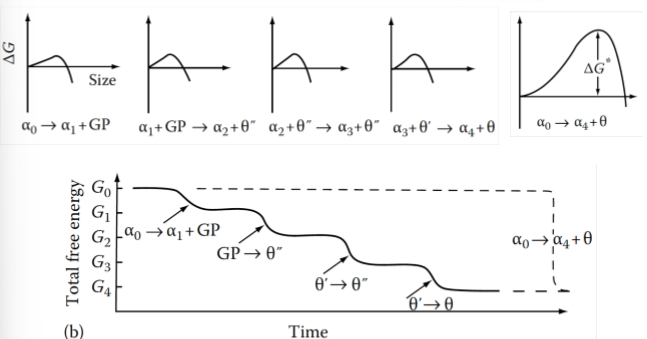
Why do transition phases form at all?
They have a lower free energy barrier
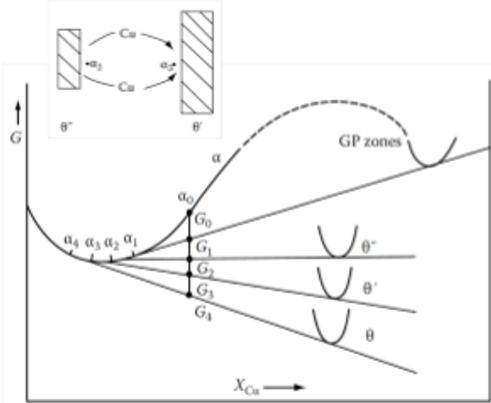
Based on the diagram, how does the θ’ phase grow?
Cu diffusion through matrix
Dissolution of θ” phase
Al diffusion through matrix
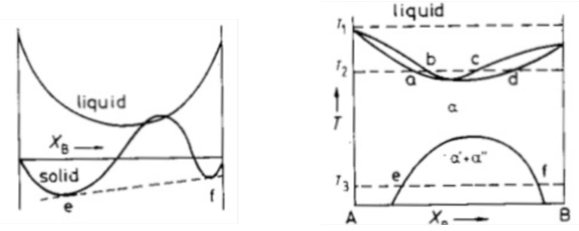
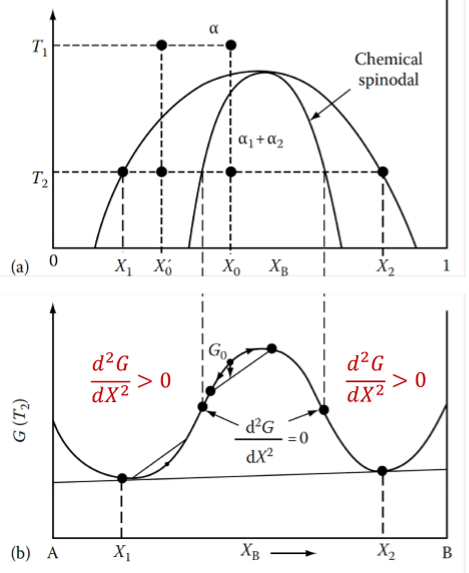
Which type of phase diagram would you expect based on the following free energy curve?
Miscibility Gap
ΔHmix > 0 → A and B atoms dislike each other and want to separate.
No barrier to nucleation for spinodal mode of transformation.
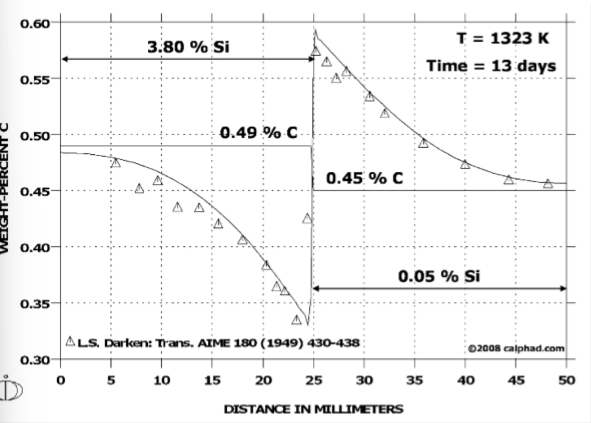
What does the concentration profile indicate is happening in the Fe-C-Si system?
uphill diffusion of C
This can happen for alloy compositions with negative free energy curvature
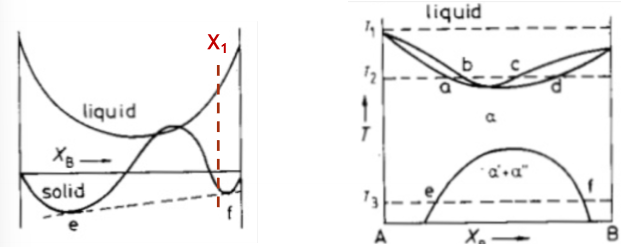
Would you expect spinodal decomposition to occur for an alloy with composition X1 indicated below?
No, never.
Spinodal decomposition with only occur in region where the double derivative is negative.
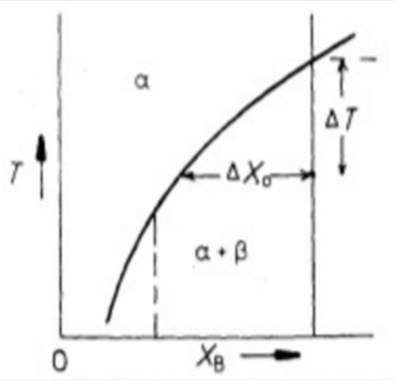
How does an increase in temperature affect the ratio of Xr/X∞ for a given particle size, molar volume and interfacial energy?
It decreasesD
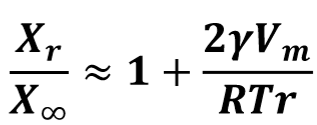
Describe Ostwald ripening
The microstructure of a two-phase alloy is always unstable if the total interfacial free energy is not minimum; therefore, high density of small precipitates will tend to coarsen into a lower density of larger particles with a smaller total interfacial area.
Nucleation vs. Growth vs. Coarsening: Does mean size of particles increase?
Nucleation: no
Growth: yes
Coarsening: yes
Nucleation vs. Growth vs. Coarsening: Is matrix at overall equilibrium composition?
Nucleation: no
Growth: no
Coarsening: yes
Nucleation vs. Growth vs. Coarsening: Is the particle number density changing?
Nucleation: yes
Growth: no
Coarsening: yes
Nucleation vs. Growth vs. Coarsening: Is the volume fraction of particles changing?
Nucleation: yes
Growth: yes
Coarsening: no
How does the rate of coarsening change if the mean radius decreases?
It increases. When grains are smaller there is a larger interfacial energy and, therefore, a larger driving force.
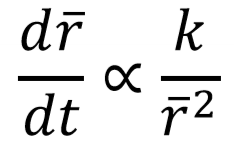
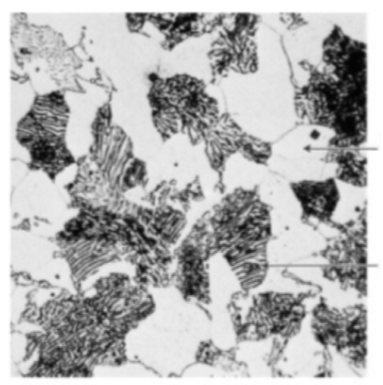
What is the C concentration (wt.%) of the alloy shown in the microstructure?
0.022 < C < 0.76
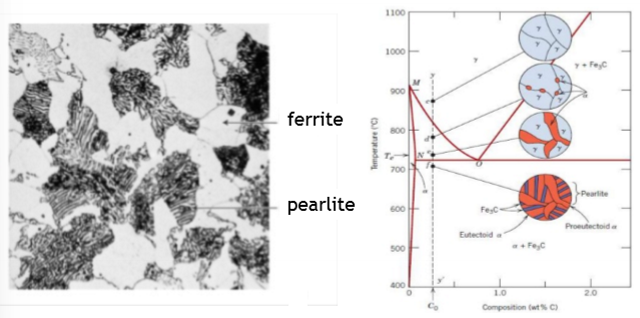
For a small undercooling (ΔT), what will the structure of ferrite look like?
Small ΔT = low driving force
Ferrite grows in a ‘blocky’ manner to form grain boundary allotriomorphs with both faceted and smooth interface.
For a large undercooling (ΔT), what will the structure of ferrite look like?
Large ΔT = high driving force
Ferrite tends to grows as plates (Widmanstatten), which become finer with increasing undercooling
When does ferrite nucleate within the austenite grains?
Sometimes
Often times things will nucleate along the grain boundaries but will sometimes nucleate within grains.
What does the presence of intergranular precipitates depend on?
Most grain size.
Describe the effect of fine grains on intragranular ferrite.
Ferrite that forms on grain boundaries rapidly raises C concentration within the grain, thereby reducing the undercooling and making nucleation even more difficult.
Describe the effect of coarse grains on intragranular ferrite.
It takes longer for C rejected from ferrite formed on grain boundaries to reach the grain centers, so there is time for nucleation to occur on less favorable sites.
What are the nucleation sites for intragranular ferrite?
Thought to be inclusions and dislocations.
What are three issues with the iron-iron carbide phase diagram?
It doesn’t show non-equilibrium phases
It doesn’t account for transformation time
It doesn’t account for cooling rate
What is the only phase that can form into martensite?
Austenite, but will usually have some retained austenite even when attempting to get 100% martensite.
Describe martensite
Supersaturated α ferrite where carbon is locked in place from quenching (rapid cooling)
Describe how baninite forms.
When austenite is cooled to large supersaturations below the nose of the pearlite transformation curve
What does the microstructure of bainite depend on?
Mainly temperature at which it forms.
At high T (350-550°C), describe the microstructure of bainite.
Upper bainite forms and consists of needles or laths of ferrite with cementite precipitates in between.
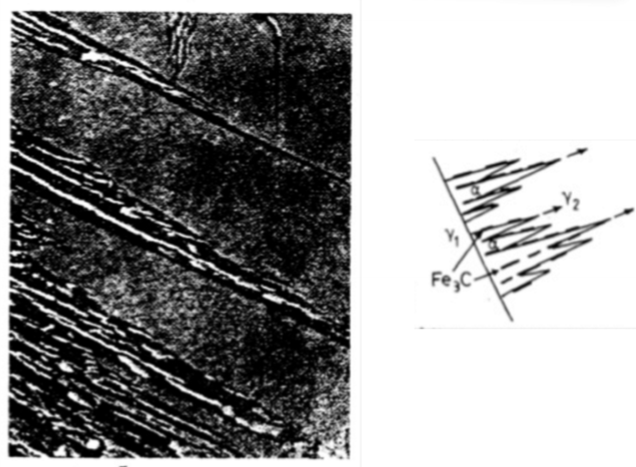
At low T (<350°C), describe the microstructure of bainite.
lower bainite forms plates rather than needles and the carbide dispersion becomes much finer.
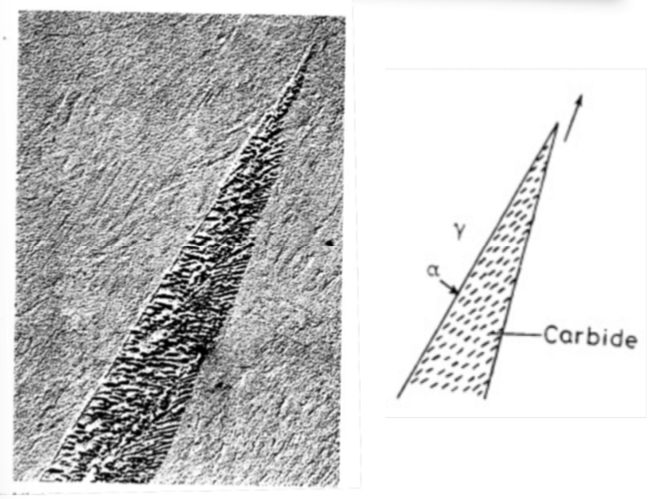
In cellular precipitation, what two ways can the growth of precipitates partition solute to the tips in contact with the advancing grain boundary?
Diffusion through the lattice ahead of the advancing cell front
Diffusion in the moving boundary
Describe a massive transformation.
For high cooling rates, no times for precipitation of one solid solution phase, so the second solid solution phase transforms into the first with the same composition. This occurs rapidly and can be defined as diffusionless civilian transformations.
What is the stoichiometry of this phase (blue = Cu and white = O)?
Cu2O
When do we get ordered structures?
Negative enthalpy of mixing (ΔHmix < 0) (i.e. prefer unlike nearest neighbors)
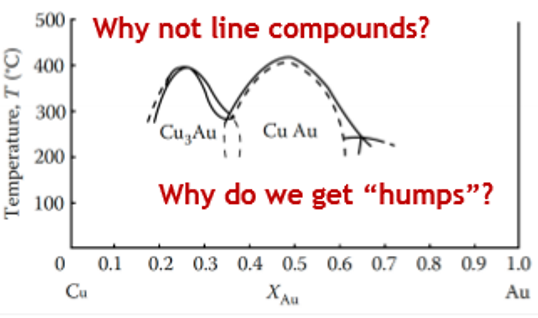
For systems with a simples A:B ratio, why do we get “humps”?
Superlattices
What are two possible mechanisms for creating an ordered superlattice from a disordered solution?
Can be continuous increase in short-range order by local rearrangements occurring homogeneously throughout the crystal which finally leads to long-range order (i.e. spinodal decomposition) and can only occur in 2nd order transformations or at very high undercooling below Tc
May be an energy barrier to the formation of ordered domains, in which case the transformation must take place via nucleation and growth (i.e. precipitation) and is the more common mechanism
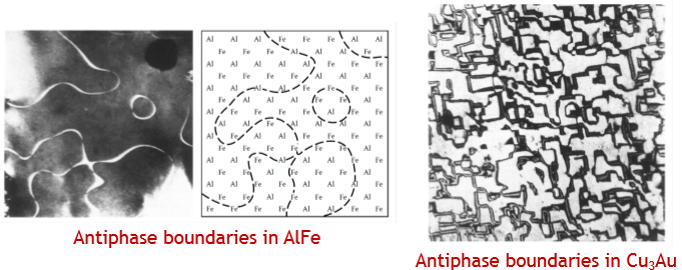
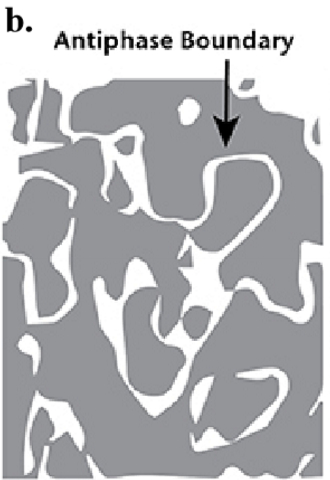
Define an antiphase boundary.
When ordered domains grow together a boundary will form across with the atoms having the ‘wrong’ neighbor
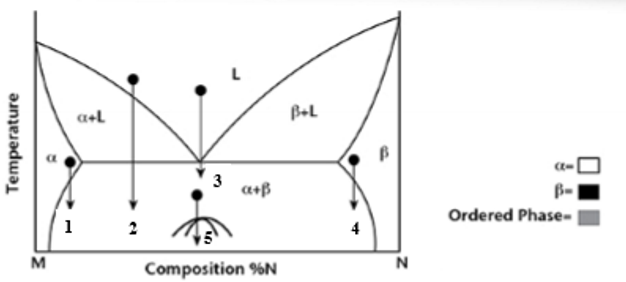
At transformation 1 on the phase diagram what is the resulting microstructure?
Precipitation - mostly α phase with small β precipitates

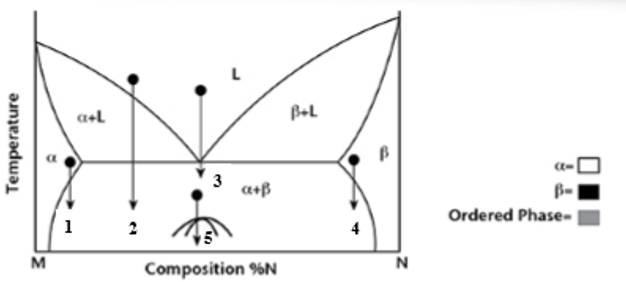
At transformation 2 on the phase diagram what is the resulting microstructure?
Proeutectic
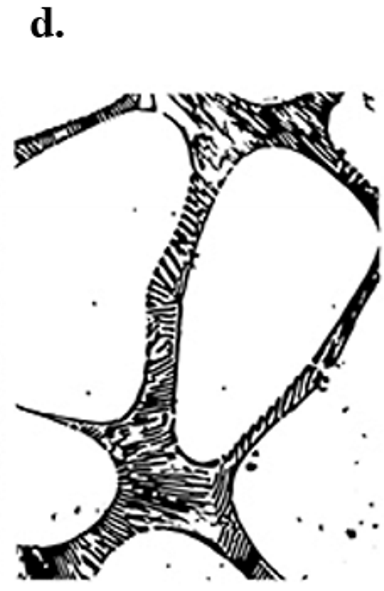
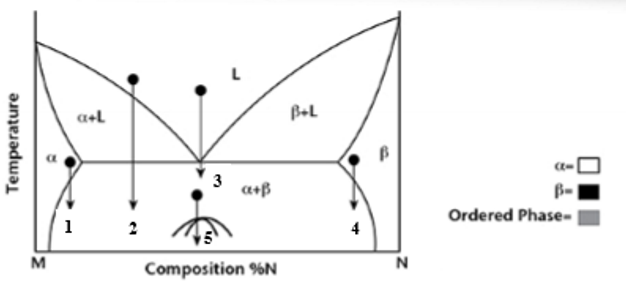
At transformation 3 on the phase diagram what is the resulting microstructure?
Eutectic - lamellar structure

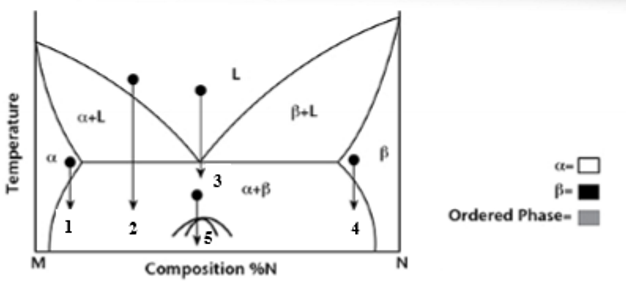
At transformation 4 on the phase diagram what is the resulting microstructure?
Precipitation - Is N rich with precipitates of α phase.

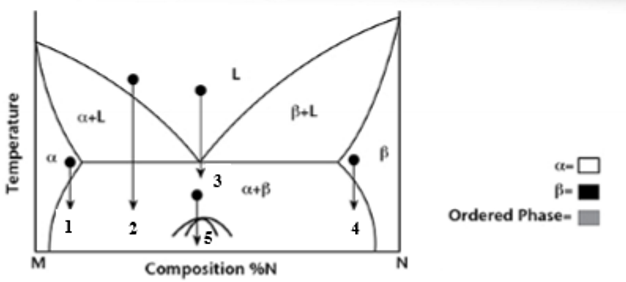
At transformation 5 on the phase diagram what is the resulting microstructure?
Order-Disorder - is below miscibility gap
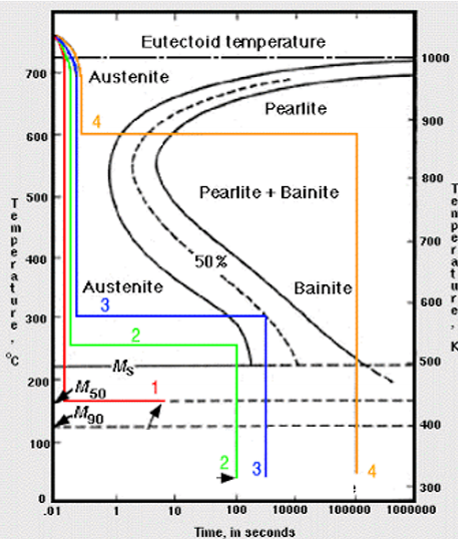
Which of the following curves should form martensite?
1, 2, 3
1 will form up to 50% martensite and 50% austenite
2 will form up to 100% martensite but may still have some retained austenite
3 can form up to 50% martensite and 50% bainite
How much austenite has transformed to martensite after cooling below the martensite finish temperature (MF)?
It depends on cooling rates.
MF is temperature below which more cooling does not increase the amount of martensite.
Austenite can be retained below MF possibly due to high elastic stresses between last martensite plates to form. ~10-15% retained austenite is common feature in higher C content steels.
What is the martensite driving force?
The martensite start temperature MS. This temperature is associated with a certain driving force for this type of diffusionless transformation of γ to α’ to occur
What 4 factors have to be considered to calculate the free energy change associated with martensite nulceation?
Interfacial energy
Interfacial area
Strain energy
Volume free energy release
Is martensite nucleation a heterogeneous or homogeneous process?
Heterogeneous
Martensite formed at higher temperatures or slower growth rates grows by what mechanism?
Slip
Martensite formed at lower temperatures and higher growth rates grows by what mechanism?
Twinning mode
How does an increase in C content affect the martensite start temperature MS?
It decreases it
Increase in C content makes it harder to form martensite.
How does grain size affect martensite growth?
Since martensite growth relies on maintaining a certain coherency with the surrounding austenite, a random high angle grain boundary can act as a barrier to plate growth. Therefore, even though grain size doesn’t affect the number of martensite nuclei, the final martensite plate size is affected.
It also affects the residual stresses present after transformation. Large grain materials have higher residual stresses built up between adjacent grains and can lead to grain-boundary rupture. Smaller grain metals tend to be more self-accommodating with smaller plate sizes and are stronger, tougher materials.
What happens upon ageing of marteniste?
Carbon segregation, precipitation of carbides, retained austenite transforms