Lecture 7: Thrombosis and Embolism
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
Thrombosis and Embolism
A thrombus is a blood clot that forms inside a vessel where it shouldn’t
A clot can block blood flow → tissue doesn’t get enough oxygen
An embolus is a piece of clot that breaks off and travels
Emboli can get stuck in another vessel and cause problems
In the lungs → pulmonary embolism
In the brain → stroke
In the heart → heart attack
Virchow’s Triad
Three main factors explain why clots form inside vessels
Endothelial injury: Damage to vessel lining makes it “sticky” for platelets
Abnormal blood flow: Stasis or turbulence slows blood and promotes clotting
Hypercoagulability: Blood is more likely to clot due to genetic or acquired conditions
Most cases of thrombosis involve more than one of these factors
Endothelial Injury
Normal endothelium prevents clotting
Releases prostacyclin and nitric oxide → stop platelets from sticking
Has anticoagulant properties (thrombomodulin, TF pathway inhibitor)
When injured, endothelium promotes clotting
Exposes collagen and tissue factor → platelets stick and clotting begins
Causes include atherosclerosis, high blood pressure, toxins, infection, inflammation
Even without rupture, “activated” endothelium can tip balance
↑ Tissue factor and plasminogen activator inhibitor (PAI-1)
↓ Thrombomodulin → less anticoagulant activity
Abnormal Blood- Stasis
Normal flow is laminar
Platelets and cells flow in the center
Plasma separates them from endothelium
Abnormal flow promotes clotting
Stasis slows washout of clotting factors and brings platelets in contact with vessel wall
Turbulence disrupts laminar flow and injures endothelium
Common causes:
Atrial fibrillation → stasis in atrial appendage
Aneurysms → turbulence and local stasis
Bed rest/immobility → venous stasis in legs
Varicose veins → stasis and superficial thrombosis
Hypercoagulability
An abnormal tendency of blood to clot due to change in the balance of pro and anti clotting mechanisms
Factor V Leiden mutation → most common inherited thrombophilia
Prothrombin G20210A mutation → ↑ prothrombin levels
Protein C, Protein S, or Antithrombin III deficiency
Pregnancy or oral contraceptives → estrogen increases clotting factors
Cancer → tumors release pro-coagulant substances
Heparin-induced thrombocytopenia (HIT) → antibody-mediated platelet activation
Antiphospholipid antibody syndrome (APS) → autoantibodies promote thrombosis
Often contributes to venous thrombosis (DVT, pulmonary embolism)
Venous clots are more likely than arterial clots
Suspect in young patients with unexplained or recurrent clots
Factor V Leiden mutation
Most common inherited form
Mutation leads to resistance to Protein C inactivation
Prothrombin G20210A mutation
Mutation in noncoding region → ↑ mRNA processing
Increased prothrombin levels → ↑ thrombin generation
Protein C deficiency
Autosomal dominant, impaired inactivation of Va, VIIIa
Protein S deficiency
Co-factor for Protein C, deficiency → hypercoagulability
Antithrombin deficiency
Impaired inhibition of thrombin and Xa
Homocystinuria
Can’t break down Methionine
High plasma homocysteine damages endothelial cells
High plasma homocysteine activates platelets
Acquired Thrombophilia
Increased tendency to clot due to medical or environmental conditions
Pregnancy and oral contraceptives → estrogen increases clotting factors, decreases anticoagulants
Malignancy → tumor cells release tissue factor and procoagulant cytokines
Prolonged immobility or bed rest → venous stasis + risk of DVT
Obesity and aging → associated with inflammation and higher PAI-1 levels
Heparin-induced thrombocytopenia (HIT) → antibodies to PF4-heparin activate platelets
Antiphospholipid antibody syndrome (APS) → autoantibodies promote arterial and venous thrombosis, pregnancy loss
Usually manifests as venous clots (DVT, pulmonary embolism)
Heparin-induced thrombocytopenia (HIT)
Immune reaction to heparin that paradoxically causes clotting
Antibodies form against heparin bound to platelet factor 4 (PF4)
Antibody-PF4-heparin complexes activate platelets
Platelet activation → thrombosis, even though platelet count drops
Onset usually 5–10 days after starting heparin
Platelet count falls by >50% from baseline
Complications include DVT, pulmonary embolism, stroke, limb ischemia, skin necrosis
Antiphospholipid antibody syndrome (APS)
Clinical syndrome caused by antiphospholipid antibodies (lupus anticoagulant, anticardiolipin, β2-glycoprotein I)
Characterized by clotting in arteries and veins, pregnancy complications, and abnormal lab findings
Recurrent venous thrombosis (DVT, pulmonary embolism)
Arterial thrombosis (stroke, MI, limb ischemia)
Recurrent pregnancy loss, stillbirth, or severe preeclampsia
Thrombocytopenia may occur
Prolonged PTT that does not correct with mixing study
Anticardiolipin antibody → false-positive syphilis (VDRL/RPR)
Primary APS (no underlying disease)
Secondary APS (commonly associated with systemic lupus erythematosus)
Deep Vein Thrombosis
Thrombus formation in the deep veins, most often legs (popliteal, femoral, iliac)
Pathogenesis (Virchow’s Triad): Stasis; Bed rest, immobility, long flights; Post-surgical or post-trauma states; Hypercoagulability
Cancer, pregnancy, oral contraceptives/estrogen therapy
Genetic thrombophilias (e.g., Factor V Leiden, prothrombin mutation)
Endothelial injury
Trauma, surgery, venous catheters
May be asymptomatic
Classic symptoms: calf/thigh swelling, pain, warmth, erythema
Unilateral edema
Pulmonary embolism (95% of pulmonary emboli arise from leg DVTs above the knee)
Chronic venous insufficiency → stasis dermatitis, varicose ulcers
Post-thrombotic syndrome → chronic pain, swelling, skin thickening
Labs: D-dimer elevate
Morphology of Thrombus
Lines of Zahn:
Alternating pale layers (platelets + fibrin) and darker layers (red cells)
Indicate thrombus formed in flowing blood (antemortem)
Arterial thrombi:
Platelet-rich, pale in color
Often form over ruptured atherosclerotic plaques
Usually occlusive
Venous thrombi:
Rich in red cells (“red thrombi”)
Almost always occlusive
Propagate in the direction of blood flow (toward the heart)
Valve vegetations:
Thrombi on heart valves
Seen in infective endocarditis, nonbacterial thrombotic endocarditis, or Libman–Sacks endocarditis in SLE
Mural Thrombus
A thrombus that forms inside a heart chamber or large artery
Adheres to the wall rather than filling the entire lumen
After myocardial infarction → damaged endocardium, akinetic wall
Atrial fibrillation → stasis in left atrial appendage
Dilated cardiomyopathy or myocarditis → poor contractility
Aortic aneurysm → turbulence and endothelial injury
Major source of systemic (arterial) emboli: Stroke, Limb ischemia, Renal or splenic infarcts
Fate of a Thrombus
Propagation:
Thrombus enlarges along vessel
May further obstruct blood flow
↑ Risk of embolization
Embolization:
Part or all of thrombus breaks off
Travels downstream as an embolus
Can lodge in lungs (PE) or systemic arteries (stroke, infarct)
Dissolution:
Fresh thrombi may be removed by fibrinolysis (plasmin)
Most effective in early stages before clot becomes organized
Organization and Recanalization:
Fibroblasts and endothelial cells grow into thrombus
Thrombus incorporated into vessel wall (scar)
New capillary channels may form → partial restoration of blood flow
Outcome depends on size, location, and balance of coagulation vs fibrinolysis
Embolism
Detached solid, liquid, or gas mass carried in the blood
Travels to a site distant from its origin
Thromboembolism:
Most common type of embolus
Arises from a thrombus (blood clot) that breaks free
Infarction: Area of ischemic necrosis caused by arterial or venous occlusion
Pulmonary embolism (PE): Embolus that lodges in pulmonary arteries; Systemic venous thrombi are the usual source
Systemic (arterial) embolism:
Embolus from the heart or large arteries
Travels to brain, limbs, kidneys, spleen, bowel
Fat/marrow embolism, air embolism, amniotic fluid embolism, tumor emboli
Always distinguish venous (→ lungs) from arterial (→ systemic organs) emboli
Thromboembolism
Most common type
Fragments of a thrombus
Venous → pulmonary embolism
Arterial → systemic infarction (brain, limb, viscera)
Paradoxical thromboembolism: A venous thrombus crosses into the arterial circulation through a right-to-left shunt (most often a patent foramen ovale), allowing a clot to bypass the lungs and cause systemic embolism such as stroke or limb ischemia
Fat and Marrow Embolism
Follows long bone fractures or trauma
Fat globules enter bloodstream
Can cause respiratory distress and petechial rash
Venous/Pulmonary Embolism
An embolus that lodges in the pulmonary arteries
>95% of PEs arise from large leg veins above the knee (popliteal, femoral, iliac)
Thrombus fragment travels → right heart → pulmonary arteries
Small PEs may be silent
Larger emboli cause sudden shortness of breath, chest pain, tachypnea, hypoxia
Massive “saddle embolus” at pulmonary artery bifurcation → sudden death
Recurrent small emboli → pulmonary hypertension and right heart failure (cor pulmonale)
Pulmonary hemorrhage or infarction (if dual blood supply is compromised)
Sudden death from acute right heart strain
Chronic thromboembolic pulmonary hypertension
Pulmonary Embolism Clinical Features
Presentation is highly variable
Small emboli → often asymptomatic
Moderate emboli → pleuritic chest pain, dyspnea, tachypnea, cough, hemoptysis
Large emboli → sudden shortness of breath, syncope, hypotension, shock, sudden death
Dyspnea (most common)
Tachypnea, tachycardia
Pleuritic chest pain, hemoptysis if pulmonary infarct
Syncope or collapse in massive PE
May show cyanosis, signs of right heart strain
Leg swelling or tenderness may suggest DVT source
Systemic Arterial Emboli
Emboli that travel in the arterial circulation and block systemic vessels
Intracardiac mural thrombi (most common, especially after left ventricular MI)
Atrial fibrillation → left atrial appendage thrombus
Valvular vegetations (infective or nonbacterial endocarditis)
Aortic aneurysms or ulcerated atherosclerotic plaques
Paradoxical emboli crossing through a PFO or septal defect
Lower extremities (≈75%) → sudden pain, pallor, pulselessness, possible gangrene
Brain (≈10%) → ischemic stroke
Other sites: intestine (mesenteric infarction), kidney, spleen, retina
Almost always cause tissue infarction
Urgent recognition and revascularization are critical to limit damage
Fat and Marrow Embolism
Usually follows long bone fractures, orthopedic surgery, or severe trauma
Fat globules and marrow elements enter venous circulation
Pathogenesis: mechanical obstruction + toxic injury from free fatty acids
Clinical: 1–3 days post-injury
Respiratory distress / hypoxemia (ARDS)
Neurologic symptoms (confusion, coma, seizures)
Petechial rash (skin, conjunctiva) from thrombocytopenia
Air Embolism
Air bubbles in circulation obstruct flow
Causes: trauma, surgery, central venous lines, obstetric procedures
Decompression sickness (divers, caisson workers)
Amniotic Fluid Embolism
Rare but catastrophic complication of labor or delivery
Amniotic fluid (fetal cells, hair, vernix) enters maternal circulation via uterine veins
Sudden dyspnea, cyanosis, shock
Followed by seizures, coma, and disseminated intravascular coagulation (DIC)
Maternal mortality 40–60%; survivors often with permanent neurologic injury
Infant also at high risk if event occurs before delivery
Infarction
Area of ischemic necrosis caused by obstruction of blood supply or venous drainage
Arterial thrombosis or arterial embolism
Occasionally venous outflow obstruction (e.g., torsion of ovary/testis)
Myocardial infarction (heart attack)
Cerebral infarction (ischemic stroke)
Pulmonary infarction
Bowel infarction (ischemia → necrosis → perforation)
Factors influencing infarction:
Nature of vascular supply (dual vs end-arterial)
Rate of occlusion (sudden vs gradual)
Tissue vulnerability to hypoxia (neurons vs skeletal muscle)
Oxygen content of blood (anemia, hypoxemia)
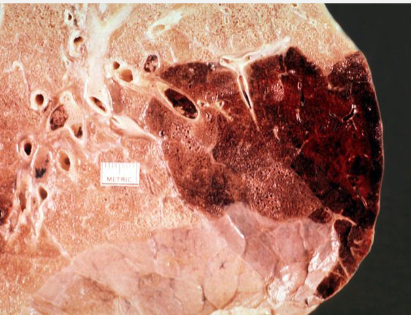
Red Hemorrhagic Infarct
Pulmonary embolism
Red Hemorrhagic Infarct
Embolism with reperfusion
Red Hemorrhagic Infarct
Loose tissues with dual blood supply (lung, intestine)
Reperfusion of an occluded artery (blood flows back into damaged area)
Venous occlusion in organs with single outflow vein (testis, ovary)
Tissue appears dark red, hemorrhagic
Blood leaks into necrotic tissue
Often associated with pulmonary embolism
Reperfusion injury can worsen damage by free radicals and inflammation
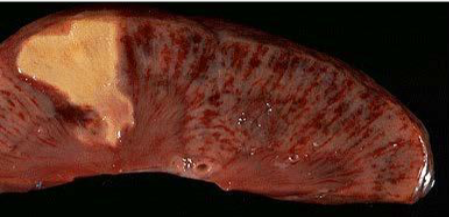
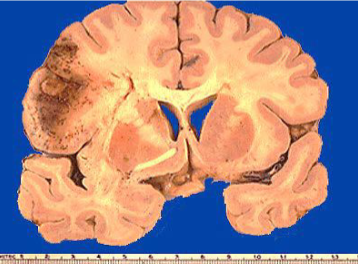
Renal infarct

White anemic infarct
Splenic infarct
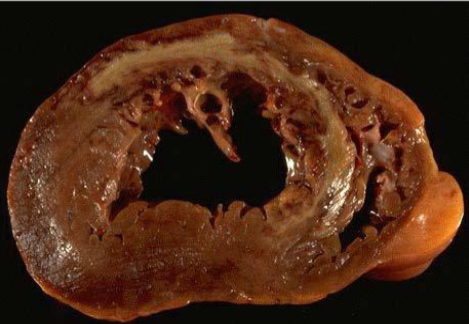
White anemic infarct
Myocardial infarct
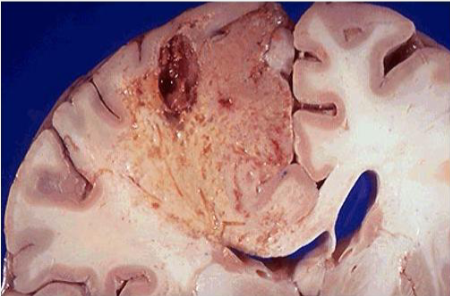
White anemic infarct
Cerebral infarct
Disseminated Intravascular Coagulation (DIC)
Systemic activation of coagulation cascade
Widespread microthrombi form in small vessels → tissue ischemia and organ dysfunction
Consumption of platelets and clotting factors → paradoxical bleeding
Excessive tissue factor release or widespread endothelial activation
Thrombin generation → fibrin deposition
Activation of fibrinolysis adds to bleeding risk
Bleeding from multiple sites (IV lines, mucosa, skin, GI tract)
Purpura, petechiae, ecchymoses
Signs of organ ischemia (renal failure, neurologic changes, respiratory distress)
Possible shock and multiorgan failure
Thrombocytopenia (platelets consumed)
Prolonged PT and PTT (clotting factors depleted)
Low fibrinogen (used up in clotting)
Elevated D-dimer and fibrin degradation products (due to fibrinolysis)
Microangiopathic hemolytic anemia (schistocytes on blood smear)
DIC Complications
Bleeding complications:
Intracranial hemorrhage
Gastrointestinal bleeding
Mucosal bleeding and hematuria
Severe postpartum hemorrhage
Thrombotic complications:
Microvascular thrombosis → tissue ischemia
Renal cortical necrosis → acute kidney injury
Liver necrosis (“shock liver”)
Adrenal hemorrhage / necrosis → Waterhouse-Friderichsen syndrome (esp. meningococcemia)
Limb ischemia and gangrene
Respiratory complications:
Pulmonary microthrombi and hemorrhage
May progress to acute respiratory distress syndrome (ARDS)
Long-term outcomes:
Neurologic sequelae after ischemia or hemorrhage
High mortality if not treated promptly
Shock
A state of systemic hypoperfusion due to reduced cardiac output or reduced effective circulating blood volume
Results in Inadequate oxygen and nutrient delivery to tissues, Cellular hypoxia → switch to anaerobic metabolism → lactic acidosis; Cell injury and death if not corrected
Hypotension (MAP <65 mmHg is typical threshold)
Tachycardia, weak rapid pulse
Tachypnea (compensatory)
Altered mental status (confusion, agitation, coma if severe)
Skin changes
Cool, clammy, cyanotic skin in most types
Exception: septic shock may begin with warm, flushed skin
Shock is a final common pathway of many lethal conditions
Initially reversible, but prolonged shock leads to irreversible multi-organ failure
Cardiogenic Shock
Failure of the heart as a pump → ↓ cardiac output
Causes: massive MI, severe arrhythmia, myocarditis, dilated cardiomyopathy
Hypotension, weak pulse, pulmonary edema, high mortality
Obstructive Shock
Blockage of blood flow despite normal heart function
Causes: massive pulmonary embolism, cardiac tamponade, tension pneumothorax
Sudden collapse, jugular venous distension, pulseless electrical activity
Hypovolemic Shock
Loss of circulating blood or plasma volume
Causes: trauma with hemorrhage, GI bleeding, severe burns, dehydration
Hypotension, tachycardia, cold clammy skin, oliguria
Distributive Shock
Peripheral vasodilation → pooling of blood, ↓ effective circulating volume
Septic shock: cytokine-driven vasodilation + endothelial injury; most common cause in hospitals
Anaphylactic shock: IgE-mediated mast cell degranulation → histamine release → vasodilation, edema
Neurogenic shock: loss of sympathetic tone after spinal cord or brain injury
Phases of Shock

Septic Shock
Organ Dysfunction in Shcok
Kidneys: Acute tubular necrosis → acute kidney injury (oliguria, anuria, ↑ creatinine)
Liver: Centrilobular necrosis → “shock liver” with elevated AST/ALT
Lungs: Acute respiratory distress syndrome (ARDS) from diffuse alveolar damage
Heart: Ischemia, arrhythmias, loss of contractility
Brain: Confusion, delirium, ischemic injury, coma in severe cases
Adrenals: Hemorrhage and necrosis in severe septic shock (Waterhouse–Friderichsen syndrome)