Topic 5 Energetics
1/5
Earn XP
Description and Tags
Section 3 (a)
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Exothermic Reaction
An exothermic reaction releases heat energy to the surroundings. Therefore, the temperature of the surroundings increases.
Examples: combustion(of hydrocarbons), many oxidisation reactions and neutralisation
Endothermic Reaction
An endothermic reaction takes in heat energy from the surroundings. Therefore, the temperature of the surroundings decreases.
Examples: thermal decomposition of metal carbonate, ammonium nitrate, …
Calculate the heat energy change from a measured temperature change
Q = mc∆T
Q = heat energy change (in Joules)
m = mass of solution/water in grams ( density of water = 1g/cm3)
*If 2 solutions are mixed, remember to add the masses of both solutions together for m.
c = specific heat capacity (usually specific heat capacity of water = 4.2 J/g/oC)
∆T = change in Temperature (in oC)
Calculate molar enthalpy change(∆H)
Find the number of moles of the limiting reagent(the reagent with the fewest no. of moles) using no. of moles = Mass / Mr
*if the limiting reagent is the solution, no. of moles = concentration(mol/dm³) x Volume (in dm³)
Divide Q( convert to kJ)by no. of moles.
Add a negative sign to the result if it’s an exothermic reaction.
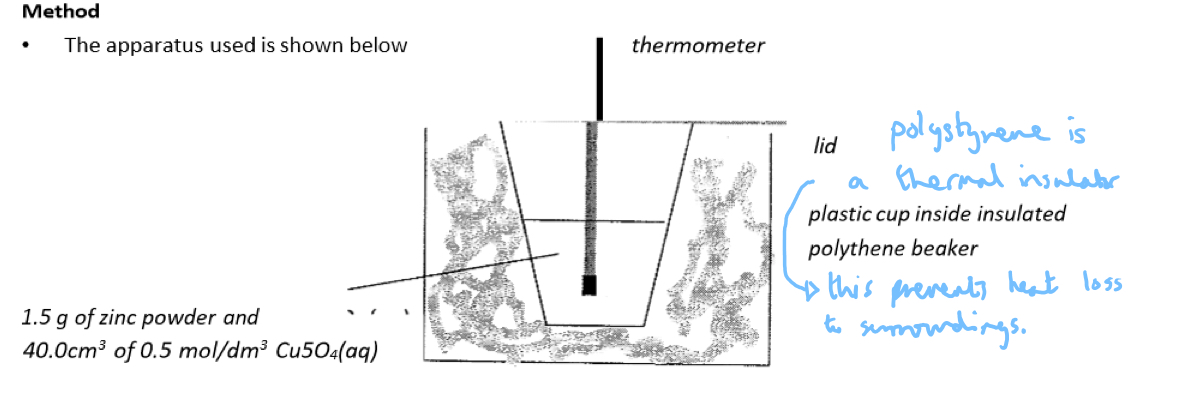
Practical 3.8: Investigate temperature changes accompanying displacement reactions, salts dissolving in water and neutralisation reactions
Key points:
Polystyrene is a good heat insulator so the polystyrene cup reduces heat loss.
The lid prevents heat loss to the air above and the surroundings.
The cotton wool provides insulation and reduces heat loss.
Temperature change in a combustion reaction
Key points
● The equation for this reaction is: C2H5OH + 3O2 → 2CO2 + 3H2O
● A copper can is used in this experiment because copper has a low specific heat capacity so only absorbs a small amount of the heat energy.
● The biggest source of error in this experiment is heat loss to the environment. This could be reduced by placing a lid on the copper can. The lid must have hole for the thermometer.