The Atom Chpt. 2
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
John Dalton - Snooker Ball model
The atom was an invisible spherical particle that made up all matter.
William Crooks Cathode Ray experiment
Showed that cathode rays are blocked by positive charges
Cathode Rays
A beam of moving electrons. They are negatively charged.
Millikan’s Oil Drop Experiment
Found the size of the electron
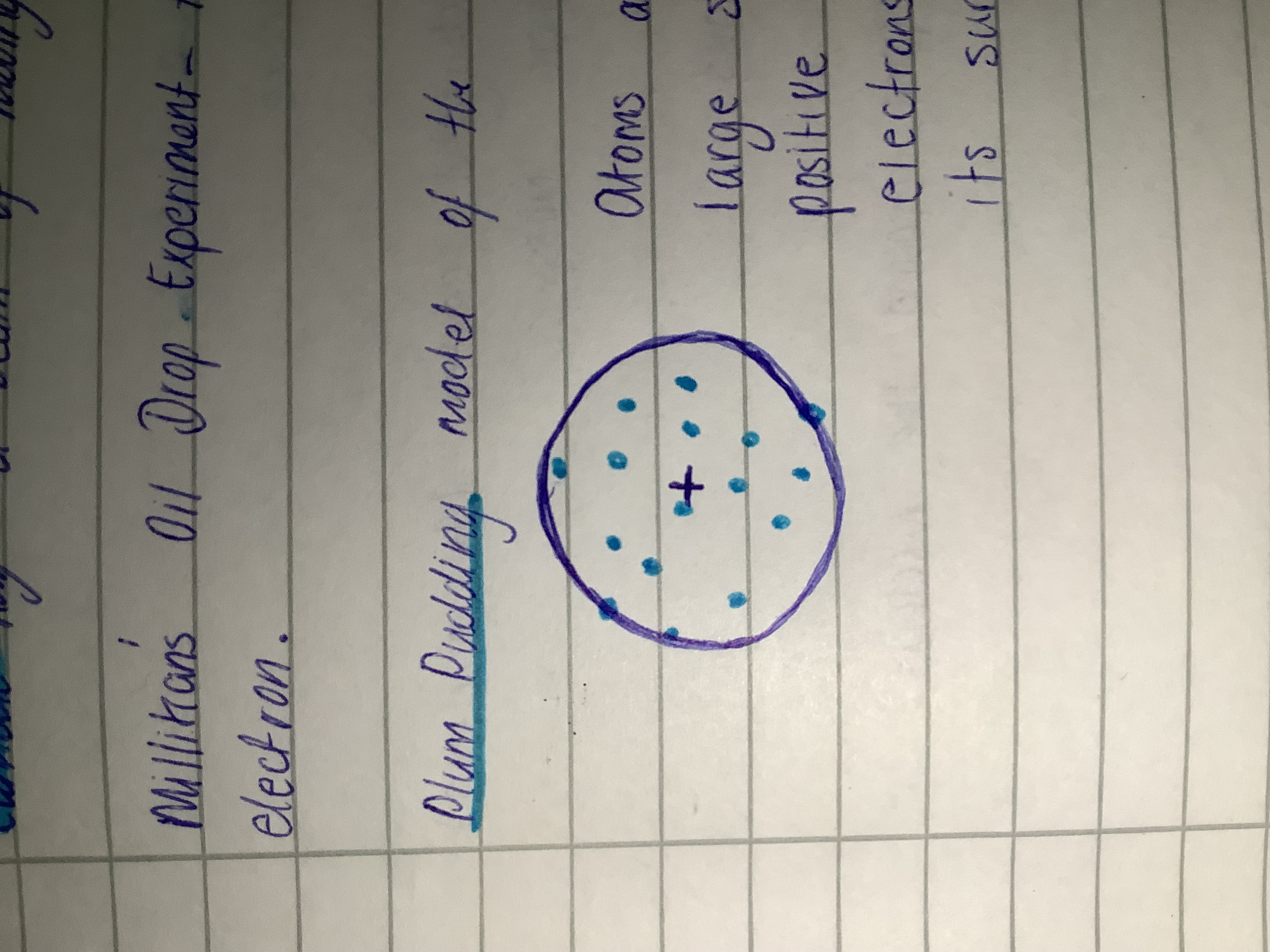
Thomson’s Plum Pudding model
Atoms are made of a large sphere of evenly distributed positive charge, with negatively charged electrons randomly embedded in its surface.
Rutherford Assumptions - Nuclear model of the atom
Atoms are mostly empty space
Atoms have a positively charged centre (the nucleus) which holds most of their mass.
Nucleus is made of protons
Electrons moved in the space around the nucleus

Rutherfords nuclear model
The atom is made of a concentrated area of positive charges (the nucleus) surrounded by a cloud of electrons.
Alpha particles
Positively charged particles made of 2 protons and 2 neutrons
Rutherfords experiment
Rutherford shot alpha particles at gold foil. Alpha particles are positively charged. After the foil was a phosphorescent screen that when struck by alpha particles, would release a slight flash. If Thomson’s plum pudding model was correct only a few particles would suffer a slight deflection. However, Rutherford found that although most particles went through some deflected at large angles and some deflected along their own path.
Rutherford nuclear model Limitations
Why don’t the positive charges in the nucleus push each other away
Why aren’t electrons pulled into the nucleus by positive charge
James Chadwick
Discovered the neutron
Updated nuclear model assumptions
Atoms have a dense, positively charged nucleus made of protons and neutrons
Most of the atom is empty space
Most of the atom’s mass is in the nucleus
Electrons are found in a cloud outside the nucleus