Carbohydrates
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What are carbohydrates - intro
Carbohydrates are organic compounds containing carbon hydrogen and oxygen
In a carbohydrate then basic unit is a monosaccharide
2 monosaccharides form a disaccharide
Many monosaccharides form a polysaccharide
What are monosaccharides and explain the types
They are small organic molecules that are building blocks for larger molecules. They have a general of (CH2O)n and there names are determined by the number of carbon atoms in the molecule Eg.
Triose sugar - 3 carbons
Pentose - 5 carbon atoms
Hexose - 6 carbon atoms
GLUCOSE - glucose is a Hexose and the carbon atoms are labelled 1-6 and share a formula - C6H12 O6 - but they differ in molecule structure (isomer). The carbon atoms of the monosaccharides make a ring when the glucose is dissolved in water and they can alter their binding to make straight chains with the rings and chains at equilibrium. Glucose has 2 isomers alpha- glucose and beta- glucose based on the positions of the OH and H ions. These result in different biological polymers of starch and glucose.
In beta glucose, on the 1st carbon atoms are labelled the hydroxide ion is above the hydrogen whereas on the alpha glucose it is the hydrogen above OH on the 1st carbon atom
Practise drawing
a triose, pentose and Hexose
Glucose a and b
Galactose
Ribose
Fructose
Practise
Give 4 functions of monosaccharides
A source of energy in respiration - carbon-hydrogen and carbon-carbon bonds are broken to release energy which is transferred to make ATP
Building blocks for larger molecules like glucose is used to make the polysaccharides - starch glycogen and cellulose.
Intermediates in reactions - triose for example are intermediates in photosynthesis and respiration
Constitutions of nucleotides eg. Deoxyribose in DNA and ribose in RNA, ATP and ADP
What are disaccharides and draw the formation of one (2 a glucoses)
They are composed of 2 monosaccharide units bonded together with the formation of a glycosidic bond and the elongation of water in a condensation reaction. The reverse is a hydrolysis reaction
Water is removed from between the C4 of one glucose molecule and C1 of another. Because the glycosidic bonds form between C1 and C4 it’s called a 1,4 glycosidic bond and due to it because straight and alpha - a-1,4 glycosidic bond.
Explain the monosaccharide components of maltose, sucrose and lactose and their biological role
Maltose - glucose and glucose - in germinating seeds
Sucrose - glucose and fructose - transport in phloem of flowering plants
Lactose - glucose and galactose- in mammalian milk
What are polysaccharides
They are large complex polymers of many monomer monosaccharides linked by glycosidic bonds and formed in condensation reactions.
Give and explain one polysaccharide in terms of structure, properties, function, components and draw them
STARCH
Structure - It is formed by converting a-glucose into starch when glucose needs to be stored appropriately. It needs to be to be stored because it is soluble in water so it would increase solution conc and lower water potential so water would enter cell via osmosis and cause cell to burst.
Properties:
insoluble in water
Cannot diffuse out of cell
Compact so can be stored in a small space
Carries a lot of energy in its C-H and C-C bonds
Found in wheat, rice, potatoes and maize
Starch contains 20-25 percent amylose and 75-80 percent amylopectin
In plants starch is the main store of glucose and starch grains are found in seeds and storage organs eg. Potato tubers
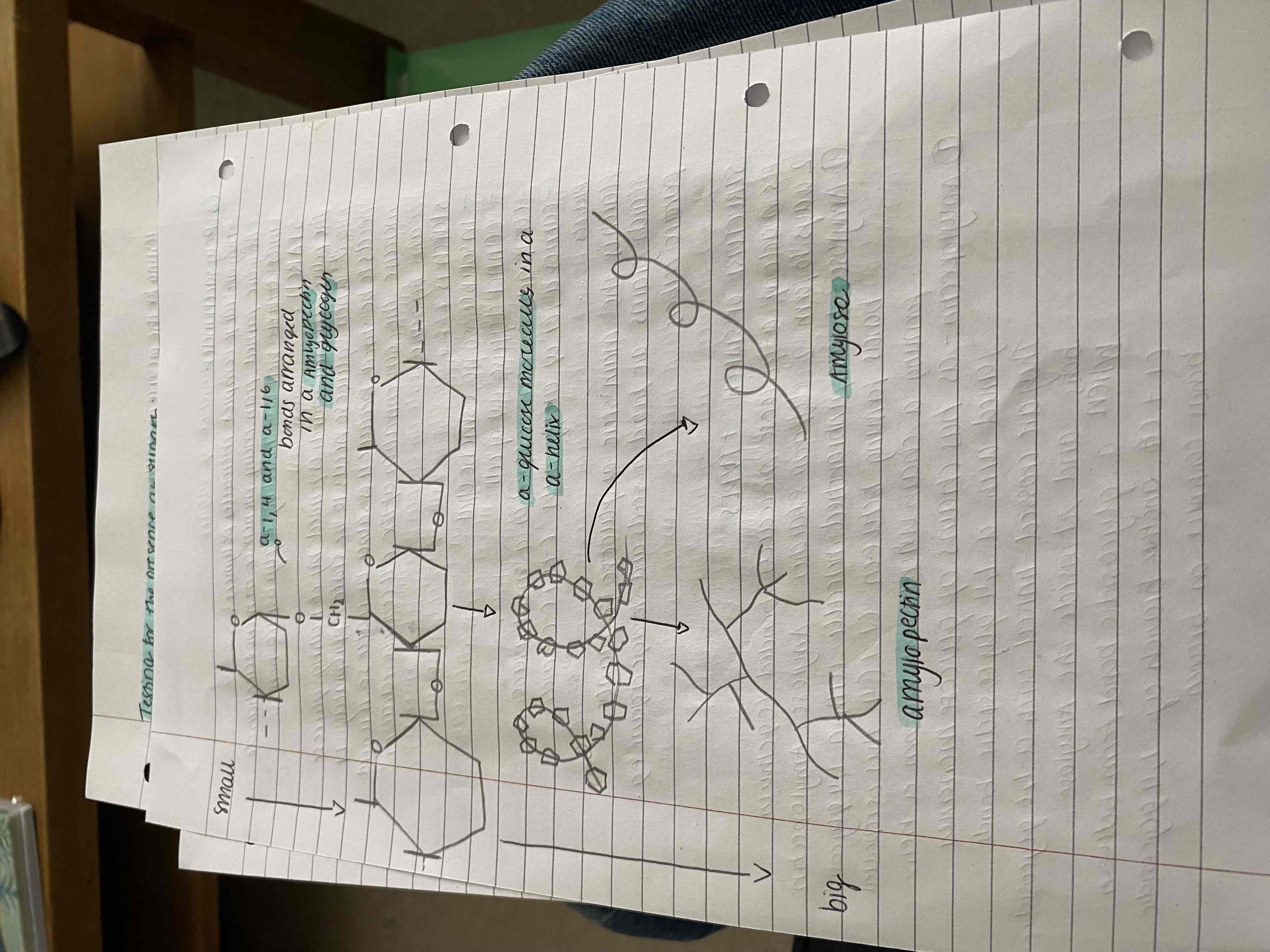
the comments of starch : the a- glucoses molecules are bonded in two ways to form two different molecules -
Amylose - linear, un branched molecules with a-1,4 glycosidic bonds. This is repeated forming a chain which coils to form a-helix
Amylopectin - has chains of glucose molecules joined with a-1,4 glycosidic bonds. They are crossed linked with a-1,6 glycosidic bonds and fit inside the amylose. When a glycosidic bond forms between c1 and c6 another 1,4 glycosidic bond continues the branch
Draw the structures of amylose and amylopectin
Give and explain one polysaccharide
Glycogen
Multi branched polysaccharide
It is the main storage product in animals
Used to be called animal starch as it’s similar to amylopectin as it also has a-1,4 and a-1,6 glycosidic bonds but the difference is that glycogen molecules have shorter 1,4 linked chains so more branches than amylopectin
It coils to a compact molecule - so they can fit many in small spaces in cell
It has a low solubility so it doesn’t lower the water potential so cell doesn’t burst
Stored in liver and Skeletal cells
It has lots of branches so lots of ends for the enzymes to easily break down the glycogen for energy.
Animals have a higher metabolic rate so they hydrolyse the glycogen bonds quicker
Give and explain one polysaccharide In terms of structure and roughly draw it
Cellulose
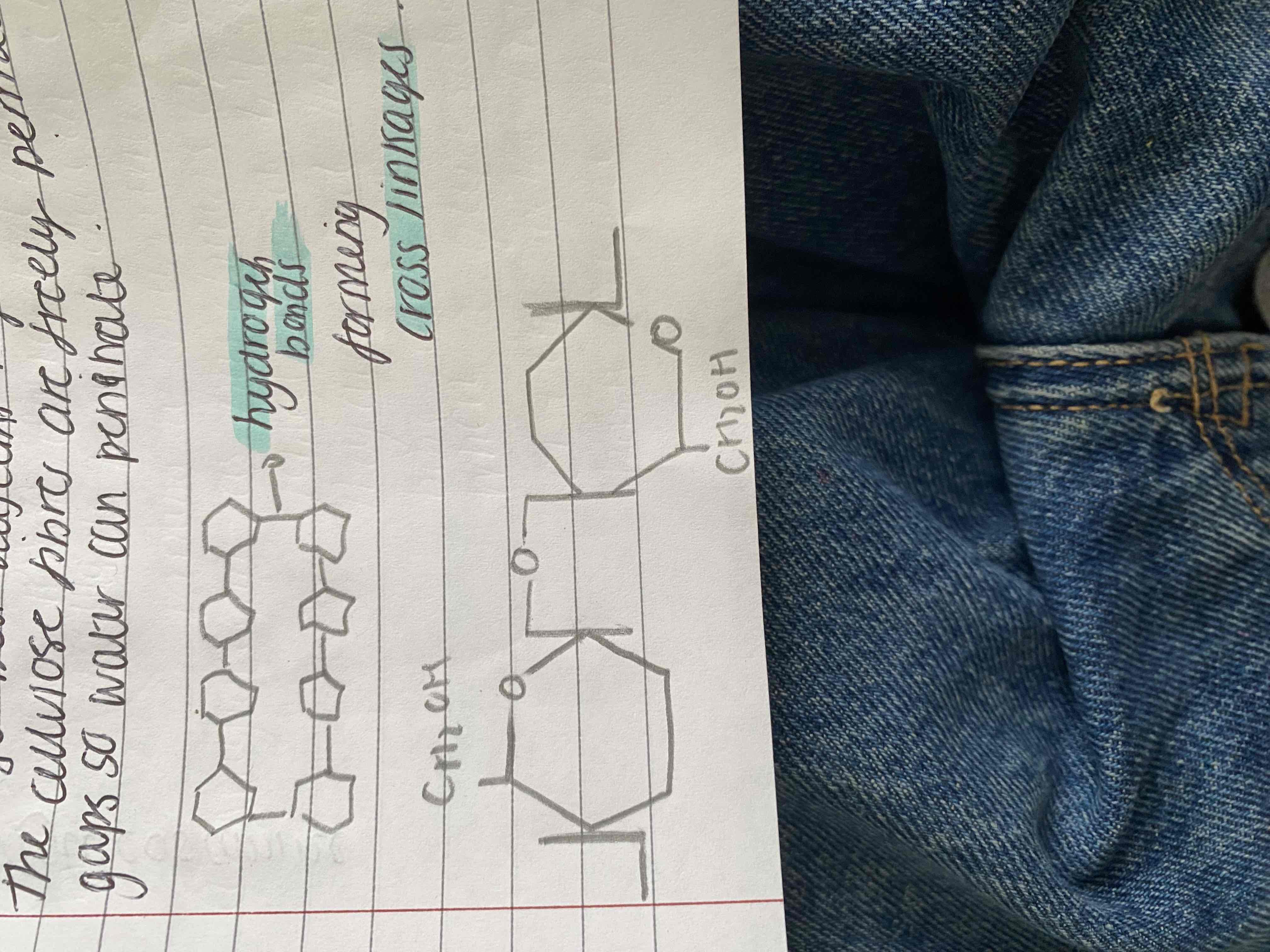
It is a structural polysaccharide and its presence in plant cell walls makes it the most abundant organic molecule on tbe planet. It consists of many long parallel chains of b-glucose units joined by b-1,4 glycosidic bonds and the b link rotates adjacent glucose molecules by 180 degrees. This allows hydrogen bonds to form between OH groups of adjacent parallel chains and contributes to celluloses structural strength. Between 60-70 cellulose mols are tightly cross linked to form bundles called microfibrils. They are held in bundles of fibres. A cell wall has several layers of fibres which run parallel within a layer but adjacent layers are at an angles
The cellulose fibres are freely permeable so water can penetrate
Give and explain one polysaccharide And draw it
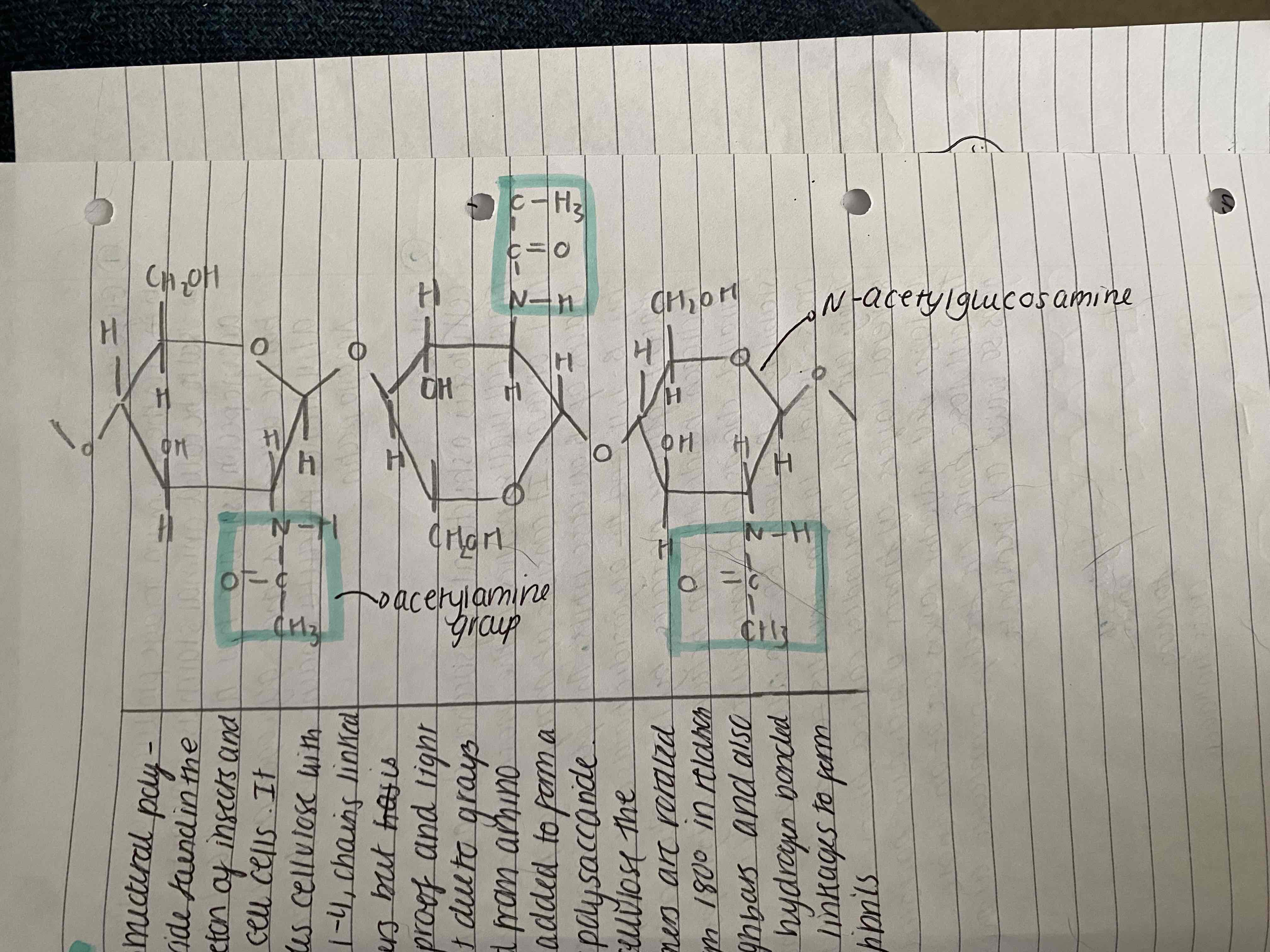
CHITIN
It is a structural polysaccharide found in the exoskeleton of insects and fungi cells. It resembles cellulose with b-1,4 glycosidic bonds in chains linked but it is waterproof and lightweight due to groups derived from amino acids added to form heteropolysaccaride. Like cellulose, molecules are rotated 180 degrees in relation to neighbours and also have hydrogen cross linkages to form microfibrils.
Explain Benedict’s test for testing for reducing sugars
Benedict’s test reducing sugars in solution
Steps
equal volumes of Benedict’s reagent and solution are heating to Atleast 70 degrees
If reducing sugars are present like glucose the solution will turn from blue to Green to yellow to orange and finally a brick-red precipitate forms. - semi-quantifiable but to give an accurate conc - biosensor (used in medicine in detecting blood glucose in diabetic patients)
this is because the sugars donate an electron to reduce copper(II) ions in copper sulphate to red copper(I) oxide
the ionic half equation is
cu2+ + e- —> cu+
Explain how to test for the presence of non-reducing sugars
Sucrose is a non reducing sugar and they give a negative result if used method above because they can’t give the electron.
First sucrose must be broken down into its constituents monosaccharides by hydrolysing the sugar using the enzyme sucrase into fructose and glucose - after that Benedict’s test can be used. Or you can add hydrochloric acid as Benedict’s solution for sucrose and non reducing sugars must be in alkaline conditions. Then heated up and brick red is positive result.
Explain the test for starch
Iodine-potassium iodide test
Iodide solution (iodine dissolved in an aqueous solution of potassium iodide ) reacts with starch resulting in colour change from orange - brown to blue-black. It’s qualitative because no conc can be determined only eye colour.