13 - Quantum physics
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
EM radiation travels through space as a continuous wave, how does it interact with matter?
when EM radiation interacts with matter, it interacts as discrete energy quanta ('packets') called photons
define 1eV
the energy transferred to or from an electron when it moves through a p.d. of 1V
what circuit to set up when experimentally determining the Plank Constant
- connect an LED of known λ in circuit with voltmeter paralell, variable resistor, ammeter, and power supply in series
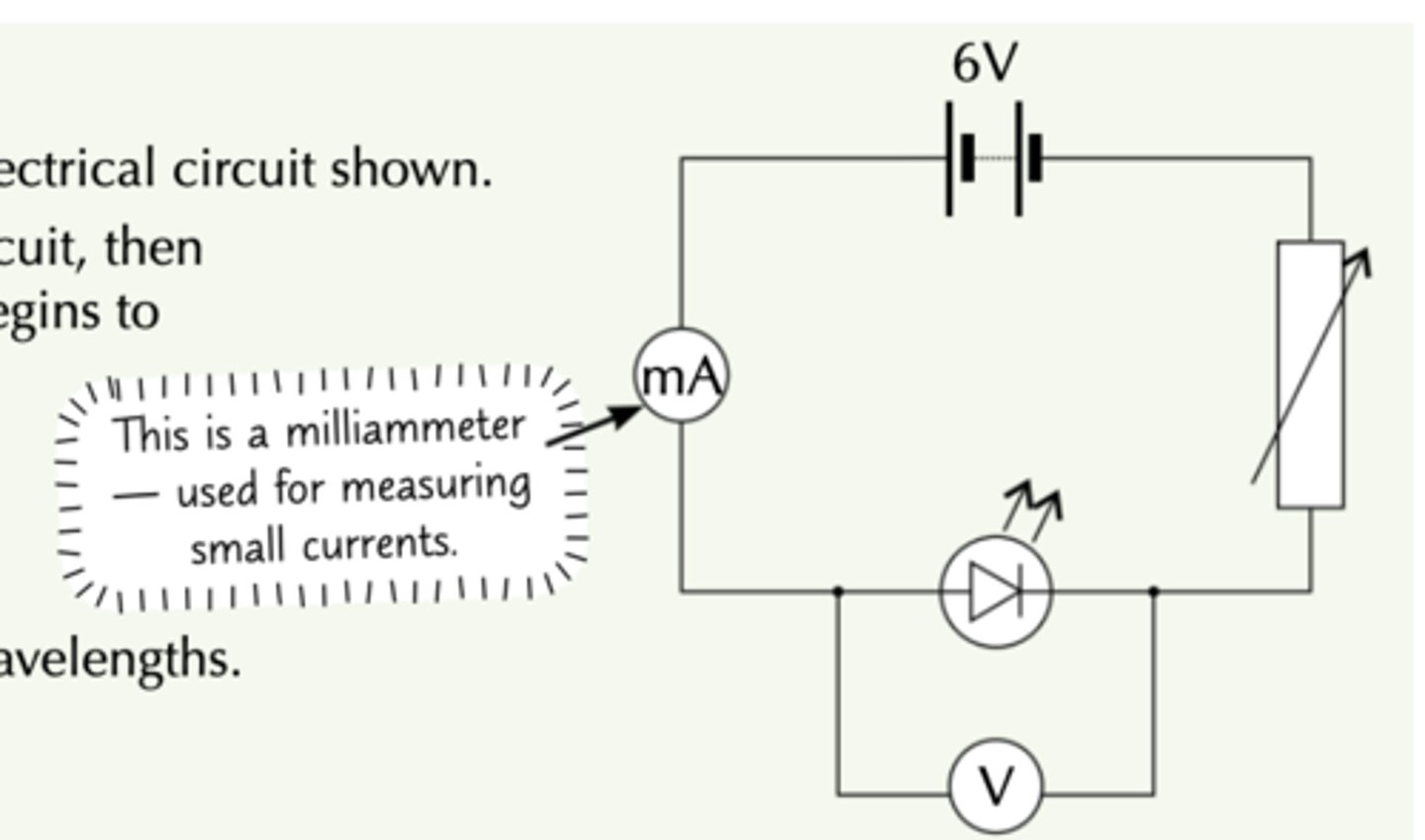
how to carry out experiment to determine plank constant by plotting a graph?
Adjust the variable resistor until a current just starts to flow and the LED lights up (place small black tube to make it obvious when LED has lit up)
- this is the threshold p.d.
Record the voltage at this point
- repeat for LED of different λ then plot all threshold voltages V against 1/λ
- gradient = hc/e
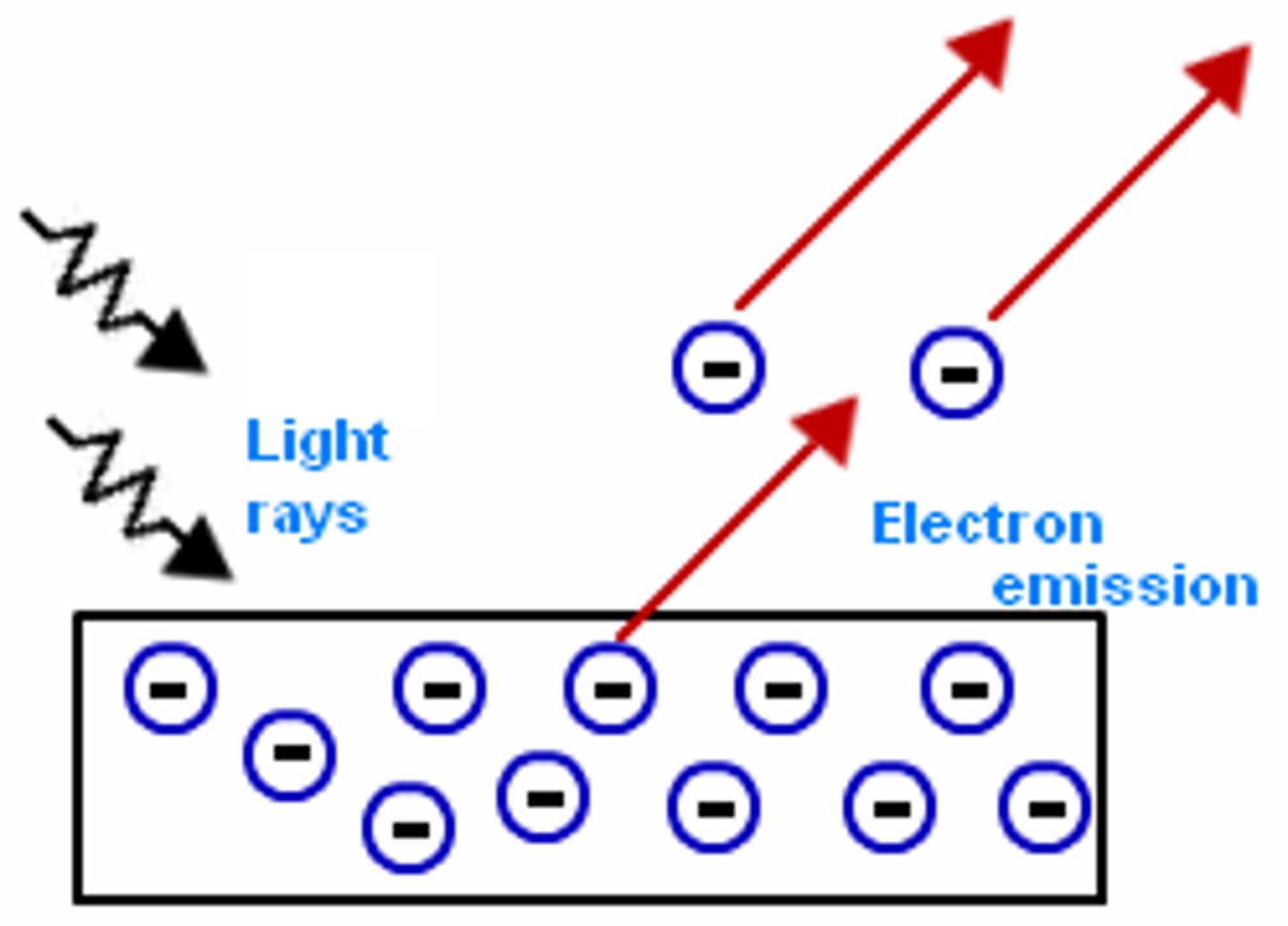
what is the photoelectric effect?
explain the process
- shining EM waves of high enough frequency onto surface of a metal will instantaenously eject electrons
- free electrons on the surface absorb energy from the EM waves, if enough energy is absorbed, a photoelectron will emit from the surface
explain how a gold leaf electroscope can be used to demonstrate the photoelectric effect
a zinc plate on top of a negatively charged stem, with a negatively charged piece of gold leaf attatched to the stem.
initially, the gold leaf and the stem have the same charge so they repel
as UV light is shone onto the zinc, free electrons from the surface will be released and negative charge will decrease, so gold leaf will fall back to stem
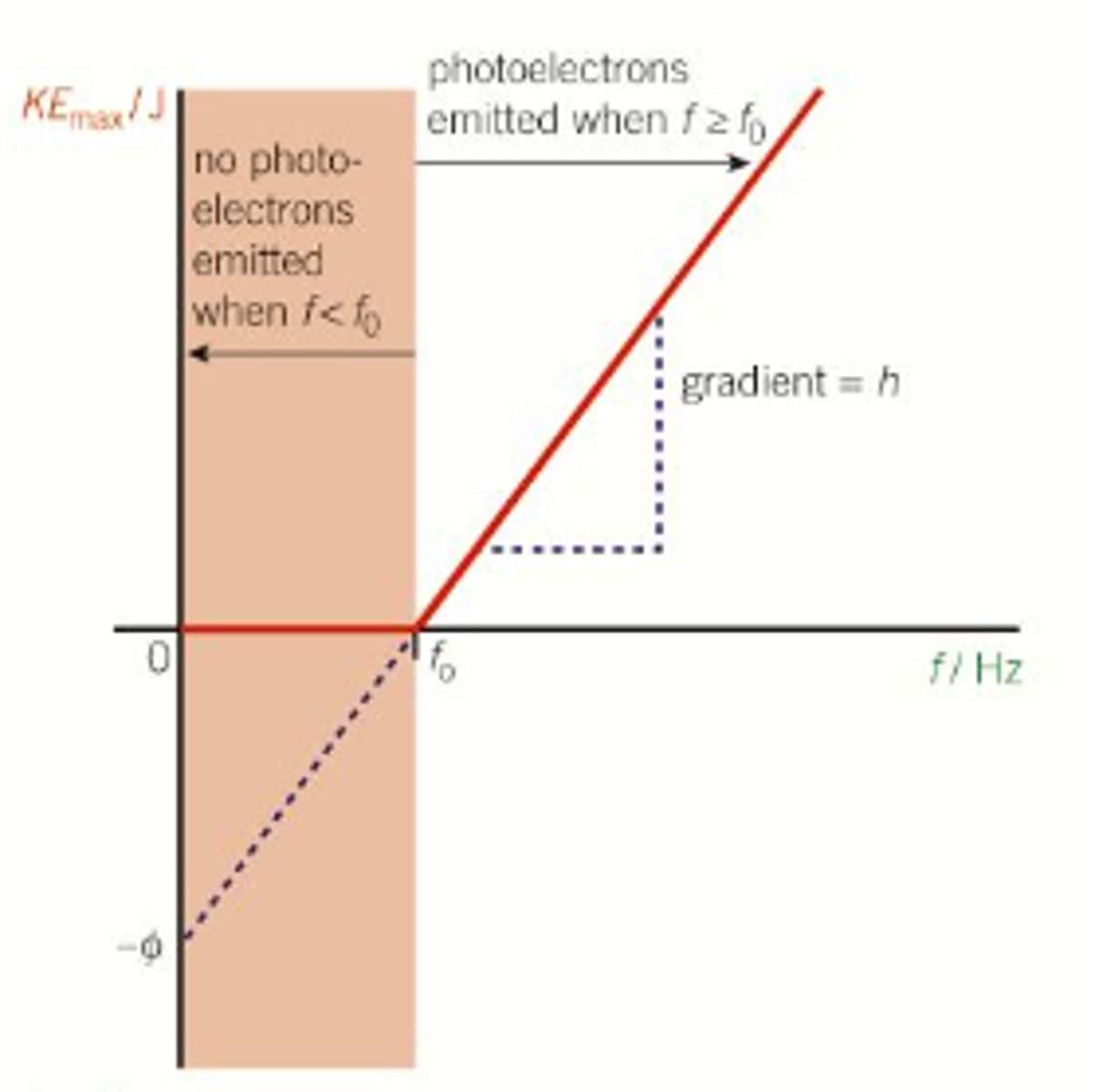
what condition is required for the incident radiation to emmit photoelectrons off a surface?
photoelectrons are emitted only if the light exceeds the threshold frequency
once threshold frequecny is achieved, are electrons emitted gradually over time due to energy build up?
once the light is above the threshold frequency, electrons emmit instantaneously, contradicting the wave model which predicted a gradual energy build-up
what effect does increasing the intensity of incident EM waves have after the threshold frequency is achieved?
what abotu before?
if light is above threshold frequency, increasing intensity increases the number of photoelectrons emitted
if not, intensity has no effect
how can you increase the maximum kinetic energy of the photoelectrons?
only the light's frequency affects the maximum kinetic energy of photoelectrons
explain the photoelectric effect
the photoelectric effect consists of EM waves incident on a metals surface
if the EM waves are above the threshold frequency of the metal, photoelectrons are instantaneously emitted in a one-to-one interaction between a photon and an electron, where the photon transfers all its energy to the electron. as the threshold frequency is met, the work functoin will be met meaning that photoelectrons can be emitted from the surface of the metal instantaneously. any excess energy is transferred to the kinetic energy store of the electrons
explain the one-to-one interaction between a photon and an electron
each photon interacts with one electron, transferring all its energy to one electron
the photon must have energy at least as great as the work function ɸ.
any excess energy is transferred to the photoelectrons kinetic energy store.
define the work function
The minimum energy required to release a photoelectron from the surface of a metal
what does increasing the intensity of the incident EM wave do?
increasing the intensity of light, when above the threshold frequency means more photons per second which increases the rate of electrons emitted, not the kinetic energy
A graph of KEmax against incident frequency
what is the concept of wave-particle duality?
how do EM waves demonstrate this?
wave-particle duality is a concept that suggests all matter and EM radiation can exhibit both wave and particle properties
EM waves demonstrate this by acting as a wave when diffracting or superposition, but also acting as discrete packets of energy called photons
What is the de Broglie equation?
what does it say about the relation between wavelength and momentum?
why is it hard to obvserve wave-like properties in larger objects
de Broglie equation:
λ = h/mv
wavelength of all matter is inversely propertional to its momentum
as momentum increases (either mass or velocity), wavelength decreases making wave properties harder to observe in larger objects
explain how electrons can diffract using an electron gun
an electron gun can fire an electron at a thin piece of polycrystalline graphite, which has carbon atoms arranged in layers
the electrons can pass between the layers, but the gap is the similar to the wavelength of the electron so the electrons diffract, forming a diffraction pattern seen on a fluorescent screen