9. Regeneration
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Regeneration
Some cells live as long as the organism itself.
Others replaced continously
Again others reformed after injury
Possibility of the fully developed organism to replace organs or appendages by growth or repatterning
•NOT linked to complexity of the organism
•
•
•
•
•
•
•Morphallaxis - repatterning instead of new
Epimorphosis
If you look at an organism like us humans, some cells in the body were generated during embryogenesis and are still present in the adult body eg many neurons and possibly heart muscle. So if you get a century old some cells in your body may also be a century old, which I think is quite amazing!! Other cells are continuously generated from stem cells, like blood and epithelia like your gut and your skin. Certain other cells relatively stable like muscle fibres, but can be regenerated when tissue is damaged. In this lecture we will look at a phenomenon which is related to this replacement: regeneration in adult organisms.
Regeneration is usually interpreted as the posibility of the fully developed organism to replace organs by growth of repatterning of existing tissue. Some organisms have remarkable powers of regeneration. For instance flatworms, starfish and hydra can regenerate an entire organism form a fragment of their body. In the case of flatworms 1/8th of animal can regenerate an entire flatworm again
An important question is why certain organisms can regenerate tissues whereas others cannot. A closer look indicates that this capacity is not linked to the complexity of the organism in question. Simple rotifers and nematodes do not have regenerative powers whatsoever. In contrast certain “complex” vertebrates like newts can regenerate surprisingly well.
We wil look at the two different types of regeneration that can occur. Morphallaxis (repatterning without growth), and Epimorphosis (regeneration by regrowth). The difference between the two types is illustrated above using the french flag as an example.
Regeneration in hydra
What is regeneration in hydra an example of?
Example of regeneration w/o growth = Morphallaxis
Hydra grows continuously, therefore cells have to change their positional values, repatterning also occurs during reproduction
-> regenerative capacity
Growth NOT required for regeneration
Head regeneration: 2 gradients
1 gradient in positional value
PV determines - head inducing ability
- resistance to inhibitor
2 head inhibitor gradient
In the next part of my talk I would like to take a more detailed look at regeneration in hydra, this is a clear example of regeneration without growth: morphallaxis.
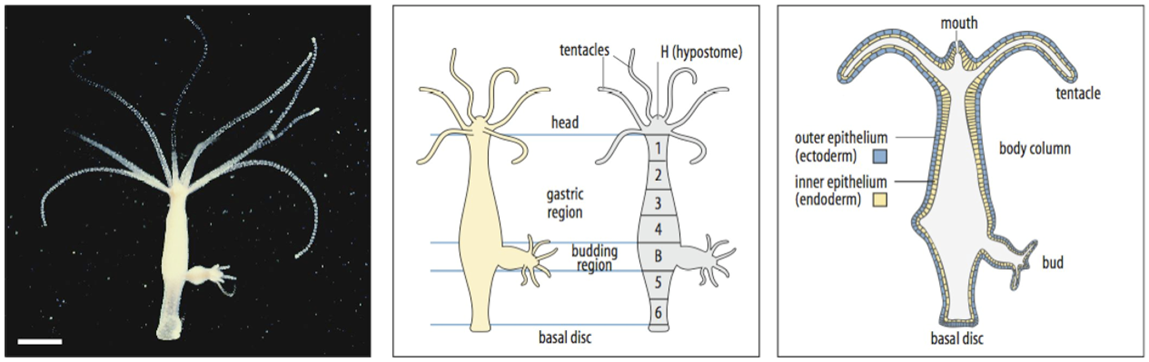
Hydra is a simple organisms that has only two germ layers ectoderm and endoderm. No mesoderm. It has a rather simple structure a mouth region called hypostome which is surrounded by tentacles an elongated body column, consisting of a gastric region, a budding region where next hydras are formed by asexual budding, and a basal disc. Hydra grows continously, and therefore in order to maintain its size, cells need to be lost. This occurs at the tip of the tentacles, at the basal disc and also by asexual budding, leading to the formation af a new individual. The fact that cells are changing their position all the time, can be visualised by labeling cells in a particlar region and following their fate. It can be shown that cells move continuously within the body, as can be seen in the lower right figure. Therefore the cells need to change their positional values continuously. In addition, repatterning also occurs during reproduction. It is thought that this contributes to the remarkable regenerative capacity of these animals.
As said before, people have done experiments that show that the formation of new cells is not required for regeneration.
When the head of a hydra is removed it is reformed not by growth but by respecifying existing cells. Numerous experiments have been performed to understand this process and these experiments have led to a model that postulates two gradients that act in this regeneration. The first is a gradient in postional value or PV. This positional value determines the head inducing ability (1 high, 5 low) and it also determines the level of resistance to a head inhibitor. This head inhibitor is produced in the head itself and forms the second gradient: the head inhibitor gradient.
Experiment to show head inhibitor
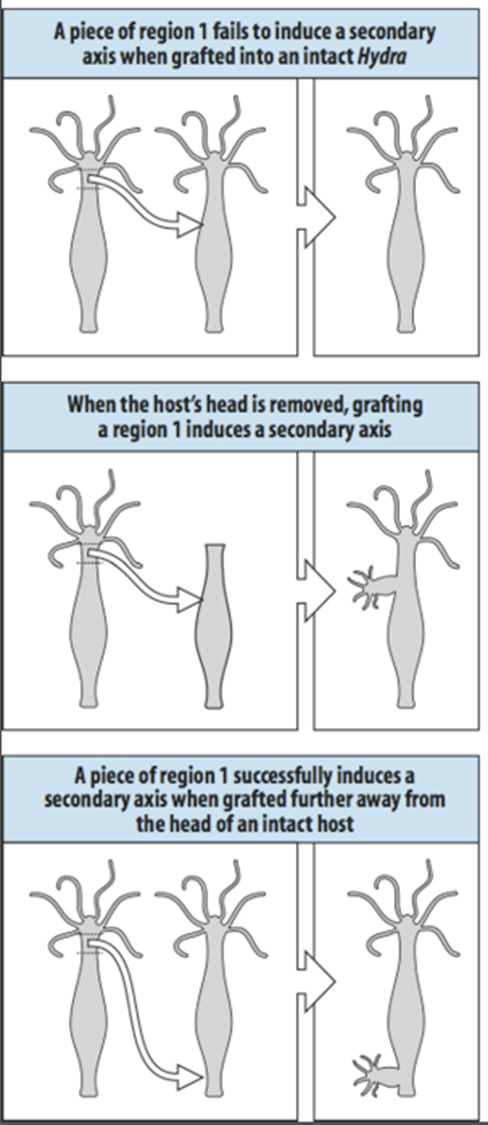
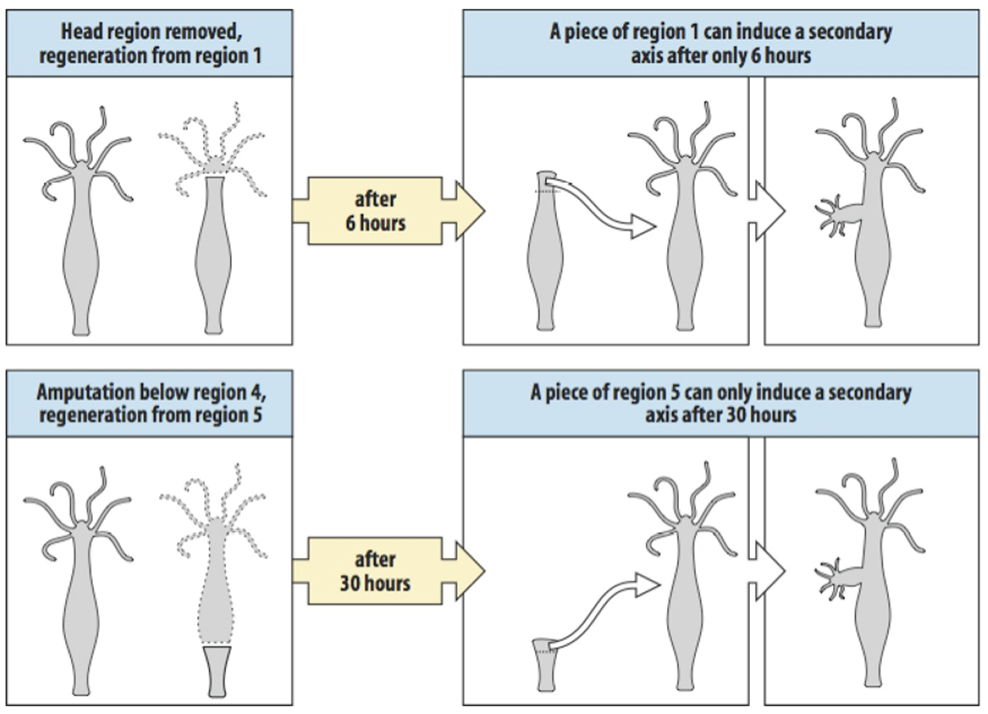
The presence of a head inhibitor can be shown by transplanting a piece of region 1 tissue to an intact hydra in the upper part of the body column, as is shown at the top of the slide. Head inhibitor from the existing head will prevent this graft from forming a second head. If the head from the donor is removed however the source of head inhibitor is lost and now a graft at the same position can form a second axis as is indicated in the middle panel. It is expected that the concentration of head inhibitor falls when tissue is further removed from the head. If piece of region 1 tissue is transplanted region 6 at the lower part of the body column, the level of head inhibitor is insufficient to prevent head formation by the graft.
Experiment illustrating: Effect of positional value/head inducing capacity
The experiments here illustrate the effect of positional value on head inducing capacity. As I said before, if you take region 1 jus below the head, and transplant it below the head of an intact hydra nothing will happen.
However if the head of a hydra is removed, and after 6 hours region 1 is transplanted into the body column of a host at a similar position as in the previous experiment, it can induce a head, showing that it has gained a stronger head inducing capacity. A piece of region five, from lower down the body column cannot do this after 6 hours, one needs to wait for 30 hours before this region has a similar head inducing capacity.
The graph at the bottom of the slide summarises happens to the two gradients after the head is removed, this model can explain all the experiments shown above.
If the head is removed the source of inhibitor is lost and this will result in an increase in positional values. In regions where this occurs the leftover tissue with the highest positional value will reach the “head-value” first and will start producing inhibitor again, thus restablishing the original situation only in a smaller body, and retaining the original polarity in the body column.
This is an example of how morphallaxis might work molecularly.
Wnt/BetaCatenin signaling may determine top PV
GSK3beta inhibition ->BetaCatenin nuclear concentration up
All regions acquire characteristics of head organiser
(shown by grafting)
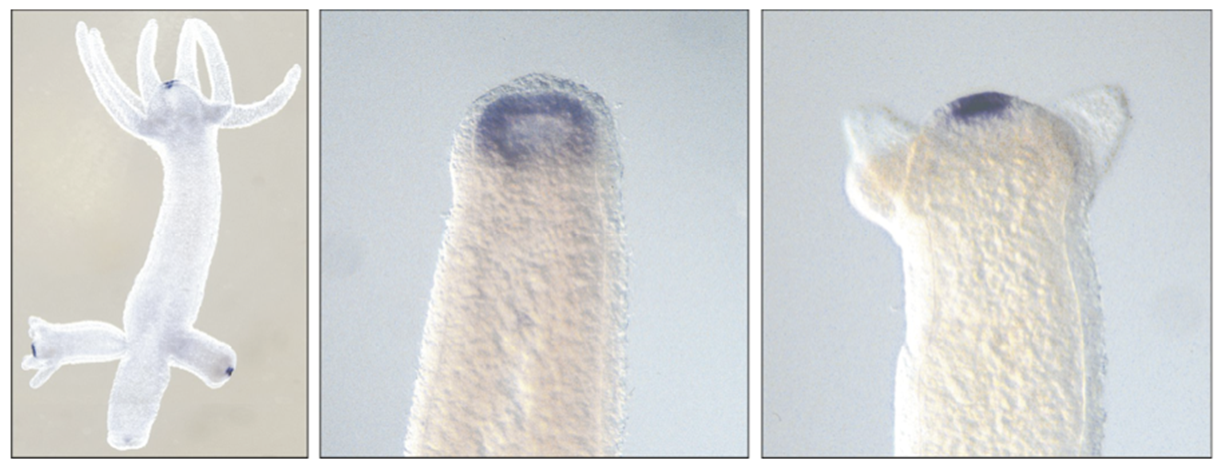
In molecular terms, the Wnt signaling is thought to be involved in head formation. Wnt is expressed in the hydra head and is also activated in a regenerating tip . Inhibition of GSK3 beta leads to upregulation of nuclear beta catenin and thereby to activation of the Wnt pathway. If you do this in hydra all regions of the body acquire characteristics of the head organiser, which can be shown by grafting.
Interestingly the xenopus spemann organiser is also dependent on wnt signaling, Hydra beta catenin is capable of inducing a secondary axis in the frog Xenopus.
Whether these organisers are clear evolutionary homologs will be difficult to prove, but
from the example of hydra it is clear that such organising centres arose very early in evolution and it is likely that vertebrates evolved from animals with a simple body plan like that of hydra
Epimorphosis
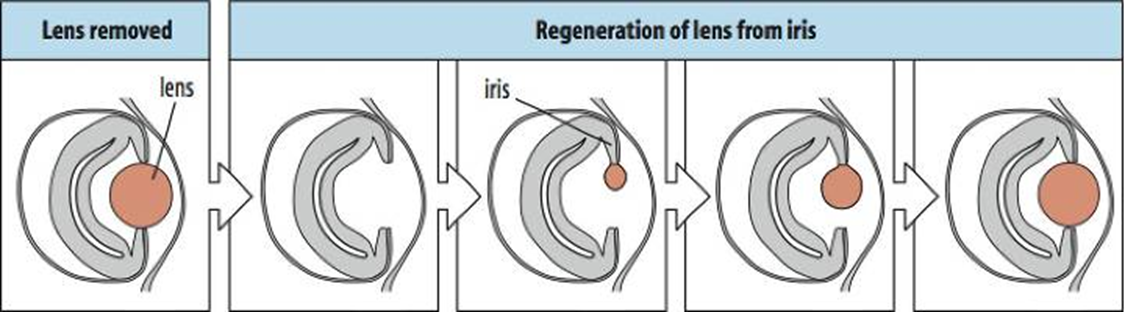
Urodele (“tailed”) amphibians are the masters of regeneration in the vertebrate kingdom. They can regenerate their dorsal crest(1), their limbs(2) their retina (3) and lens(4), their jaw (5) and their tail. Interestingly lens regeneration occurs from the iris as is shown in the bottom panel, thus cells need to transdifferentiate.
An example of limb regeneration is shown on the right. As you can see regeneration occurs from a level that is approriate to where the cut was made, a distal amputation only regenerates distal structures, whereas a proximal amputation also regenerates proximal structures. So the tissue seems to know which tissues need to be reformed.
In the case of the newt, all this regeneration occurs by growth of new tissue, therefore it is of the epimorphic type. If you take the limb as an example, regeneration involves production of various tissue types like bone, muscle, blood vessels, and skin. So an important question concerns the origin of the cells, where to they come from??
Are there special cells that are set aside for regeneration (a quiescent stem cell) or is it done by dedifferentiation of particular cell types. The origin of the regenerate is a really interesting question.
Epimorphic
Limb:
Various tissues
Origin??
Urodele limb regeneration
After amputation: epidermal cell migration,
Regeneration is dependent on this.
Cell below the epithelium dedifferentiate; blastema
dermis, cartilage, muscle
Thrombin (protease)
Msx1
pRb phosphorylated (inactive)
Generation/regeneration:
different scale
Very limited transdifferentiation
Dermis->cartilage
Rest true to type
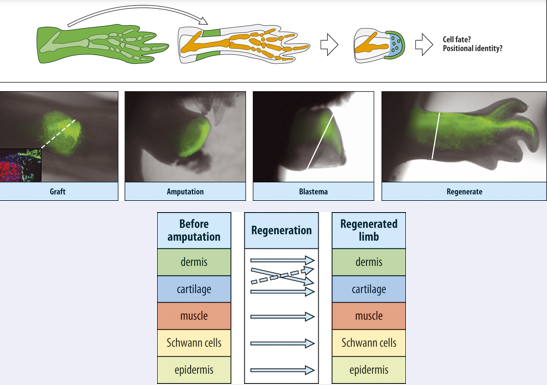
Lets look at amphibian/uordele limb regeneration in more detail. The first thing that happens fter amputation is that the epidermal cells migrate over wound surface, this is essential for regeneration to occur.
To continue with the process of regeneration once the epithelium has covered the wound, cell below this epithelium dedifferentiate and form a blastema
The blastema is derived from the dermis but also from dedifferentiating muscle and cartilage.
The fact that the muscle can also participate is quite surprising since muscle cells are multinucleate. It has been found that multinucleate muscle cells can revert to mononucleate cells in cell culture under the influence of thrombin, which is a protease. Dedifferentiation of muscle cells involves expression of msx a homeobox transcription factor, and inactivation of the Rb gene (by phosphorylation), which inhibits proliferation of muscle under normal circumstances. The presence of thrombin is is crucial for dedifferentiation and may have wider significance, lens regeneration also requires local activation of thrombin.
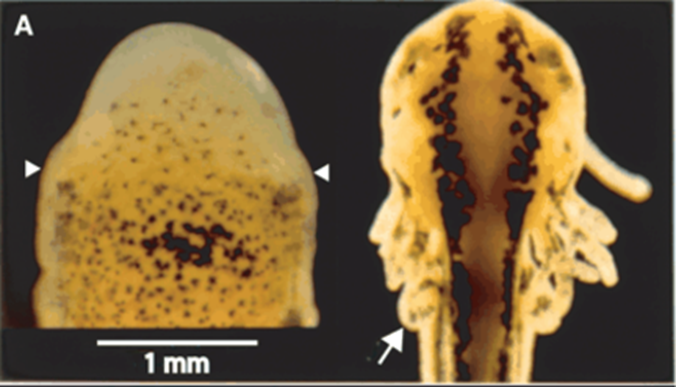
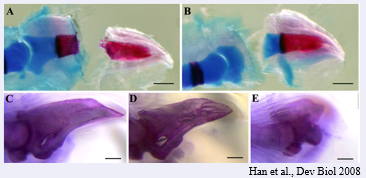
It is important to realise that generation of a limb during embryogenesis and regeneration must be different, the figure over here shows a limb bud on the right (white arrow) and a regenerating limb (between white arrowheads on left) , as you can see in the figure, the scale is quite different. Thus if morphogens are involved, they somehow need to work over a 10x larger range, it is hard to imagine how this would work molecularly, although there may be lots of parallels, there must also be differences.
An interesting question that can be posed is that if cells are dedifferentiating, to what extend are they dedifferentiated, if they were muscle cells can they suddenly give rise to dermis? Or can a dedifferentiated cartilage cell give rise to muscle? People have done experiments to look at this and the consensus is that there is very little transdifferention, dermis cells appear to be able to give rise to cartilage but the rest is, to all intents and purposes, true to type, so dedifferentiation is very limited.
So the blastema provides a only limited dedifferentiation environment. An experiment illustrating this is shown in this slide . Here they took tissues from a GFP labeled animal and transplanted, for instance, the dermis to the limb on an unlabeled animal. When the transplant had taken they amputated the limb at the level of the transplant, and looked what the labeled cells were contributing to. Were they only forming dermis or were they also contributing to other tissues in the regenerated limb.
When they did such experiments on a larger scale they found the results shown in the table below…
There was a bit of celtype change between dermis and cartilage, but all others were true type, muscle form muscle, schan cells form schwan cells and epidermis forms epidermis.
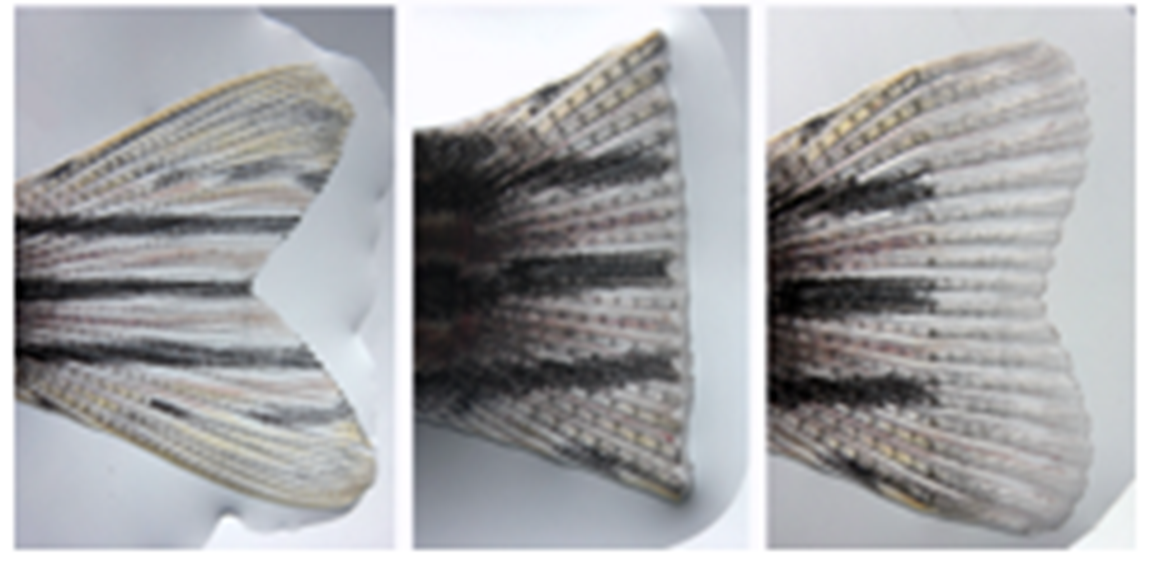
In fin regeneration cells are true to type
Similar experiments have been performed in a different organism the zebrafish using fin regeneration, and here the same observations have been made.
“Rules of regeneration”
•Limb regeneration is always distal to the wound
•According to positional value at site of the cut
•not just replacing missing parts
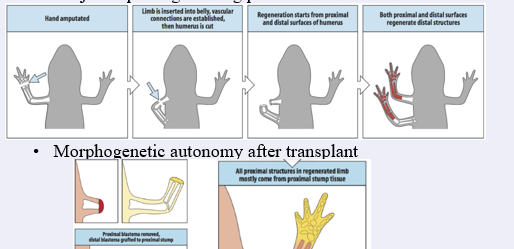
•Morphogenetic autonomy after transplant
According to what rules does regeneration occur? First of all regeneration is always distal to the wound, and, importantly, according to the positional value at the site of the cut. It is not a process that tries to just replace missing parts.
This can be illustrated by some surprising ”outcomes” as is illustrated by the experiment in the middle of the slide:
If the distal limb of a newt is amputated and the stump is inserted into the flank (to establish vascular connections) and the limb is subsequently cut again, the anterior limb will regenerate the missing distal parts as expected. Importantly, the remaining stump will also regenerate a distal limb, with a mirror image humerus, and not a proximal humerus,.
So, the wound blastema “reads” the local positional value and generates more distal positional values, it does not determine what is missing. So as a result a limb with two radii and ulnae is formed.
A blastema also has considerable autonomy, once formed it can regenerate the structures lost in a different location (dorsal crest for instance).
This will also happen if a distal blastema is tranplanted to a proximal wound as shown in the lower picture on the slide The “yellow” blastema wil only form the hand. Nevertheless, an entire limb will form. This shows that the site of the wound can sense a discontinuity in positional values between the distal blastema and the cut site. The remaining tissue will be formed by intercalary growth from tissue that is just below the transplantation point
Molecular/Cellular basis
Proximal and distal blastema cells may sort via
differential adhesion
Retinoic acid (RA) can proximalize a blastema
via Rarδ2, meis homeobox genes and/or
upregulation of a GPI linked protein prod1
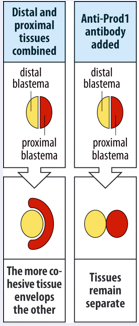
What is the molecular/cellular basis of this positional value. This is not known for sure but there is an indication that differential adhesion is important. If a distal blastema (yellow) is combined with a proximal blastema (red) the proximal blastema will start to engulf the distal blastema as illustrated on the right. This can be interpreted as a sign the distal cells stick more tightly to one another and that the proximal cells stick more to the distal cells than to themselves.
Differential adhesive properties might also help to explain why distally transplanted cells do not mix with the proximal blastema cells that will form as a result of the intercalary regeneration which I mentioned before.
It is unclear how positional information is encoded in the blastema, but retinoic acid (RA, lower right) may be involved in settings these values. It has been found to be capable of resetting this positional value to a more proximal value. The effect of RA is dose dependent . The higher the level the more proximal structures will be regenerated.
The effect of RA is shown in the figure on the lower left. Here a hand was amputated and after amputation the blastema was treated with a high concentration of RA, this led to a proximalisation of the blastema and it went on the regenerate an entire limb at the level of the wrist.
RA works in a concentration dependent manner
Proximalisation by RA may occur via RARdelta2 and upregulation meis homeobox genes and/or prod 1. Prod1 is a gpi linked adhesion protein that is expressed at the highest level in the proximal regenerating blastema, and is induced by RA treatment. Prod1 may play a role in the differential adhesion we discussed. This was indicated by the experiment shown at the far right. Anti Prod 1antbodies could prevent the engulfment that is normally seen when proximal and distal blastemas are apposed.
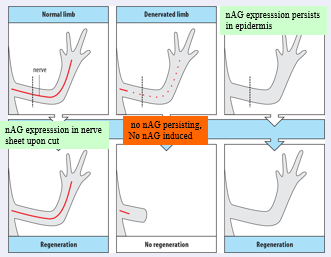
In a normal limb innervation is required for regeneration to occur, except if the limb had no nerve in the first place
-Newt “Anterior Gradient” (nAG) is a protein that can replace the nerve in supporting outgrowth, binds prod1
-Expressed in nerve sheath in response to wounding
Innervation leads to downregulation of epidermal nAG
Another rather curious fact about limb regeneration:
Regeneration is dependent on innervation, unless the limb has without a nerve from early on in development.
This is illustrated in the schematic above.
This recently this rather unusual observation got a molecular explanation: it was shown that a molecule called “newt anterior gradient” or nAG can “rescue” regeneration of a denervated limb. Interestingly nAG is a ligand for prod 1 and is expressed by the nerve sheath and the epidermis, both expression domains are dependent on innervation.
What was found is that innervation leads to downregulation of epidermal nAG but when such an innervated limb is cut the nerve sheet expresses nAG. If a limb is denervated before the amputation this induction does not happen. In a continuously denervated limb as is shown on the right, epiderman nAG is maintained, this suffices to promote regeneration.
Insect regeneration-cockroaches
Sensing discontinuities in positional values
Intercalation to regenerate missing ones
Irrespective of overall structure
Segments of a leg contain similar positional values
->Graft of a mid tibia to mid femur will not cause regeneration of
Intermediate structures
Certain insects can also regenerate their appendages, This has been studied in cockroaches in more detail, and the mechanism also involves sensing of discontinuities in positional values, missing positional values are then filled in, So principles appear to be similar to what happen in newt limb regeneration.
Again, regeneration happens irrespective of the overall structure.
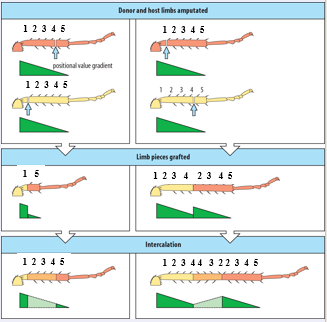
It has been found that each segment of he leg contains a similar set of positional values. This means that if a mid tibia is grafted to a mid femur NO regeneration will occur.
An example of a grafting experiment that illustrates the principle of regeneration is shown here.
The donor leg is coded brown and the receiving leg is coded yellow. In green a gradient of a hypothetical positional value is shown. If positions 1 and 5 are opposed the intermediate values are generated and a normal leg is restored. If the transplant is done like in the left panel where positional value 2 is grafted distal to positional value 4, again the missing positional but this values are intercalated. Thus a segment of leg is formed with reverse orientation. It does NOT delete the duplicated positional values. So the system does not know what the overall situation is, it just responds in a fixed way to discontinuities in the positional values.
Mammalian regeneration
Young children/mice can regenerate tips of their digits
Peripheral nervous system, regrowth of axons, not neurons,
Schwann cells
CNS very little regeneration non permissive environment (CNS glia:
astrocytes, oligodendrocytes)
PNS schwann cells can promote axon growth in the CNS
Liver, ribs
Mammals are not good at regeneration but not fully incompetent…
Young children and mice can regenerate the very tip of their fingers. Tis illustrated in the picture, if a fingerthip is amputated distal to the nail bed the tip can regenerate as shown in D, however if the nail bed is also removed regeneration will fail.
Furthermore in the peripheral nervous system axons can be regenerated, however if the neuron itself is lost no regeneration occurs. Regeneration occurs along the orignal pathway of the axon and is stimulated by schwann cells along the way.
In the CNS little regeneration occurs, neurons actually try to send out new axons but they appear to collapse it is thought that astrocytes and oligodendrocytes of the CNS provide a non permissive environment. It has actually been found that implanted schwann cells in the CNS can promote axon growth.
One assumption that may explain this difference is that the CNS is a very delicate and elaborate system that could be disrupted easily if new axonal could form without difficulty.
Other tissues which can regenerate are the liver, this organ can be reformed when is is partially resected.
Als ribs have been reported regenerate as long as the periosteum, the membrane that surrounds the ribs is left intact.
The latter is often exploited by surgeons doing reconstructive surgery, ribs can be source of transplantable bone material.
Vertebrate Heart regeneration
Mammals: Cardiomyocyte are present but don’t divide; Progenitors present, but not used?
Scar/maladaptive hypertrophy
Ventricular regeneration in zebrafish
Not identical to embryonic heart development eg.
msxB and C expressed
Regeneration dependent on dedifferentiating muscle cells
Endocardium /Epicardium are involved
Neuregulin may be a signal from the epicard, inducing proliferation in myocard
Scarring vs regeneration
zebrafish mps1 mutants show scarring rather than regeneration
Due to its importance for human health. I would also like to touch on vertebrate heart regeneration. Cardiac cells in the mammalian heart hardly divide.
More recent work on mammalian hearts suggests that progenitors that can differentiate into muscle may in fact be present, but somehow they are not used when tissue is damaged. Rather, necrotic tissue that forms after infarction is replaced by scar tissue. In addition, there is a hypertophic response of the remaining muscle cells, which is thought to be maladaptive, and may in fact increase the risk of further infarction.
In zebrafish however the ventricle of the heart can be regenerated. Due to differences in scale and also by looking at molecular markers it is clear that this regeneration is not identical to embryonic heart formation. For instance, Msxb and Msxc are expressed during regeneration but not in development.
Recent data indicate regeneration is dependent on dedifferentiating muscle cells, and is not driven by stem cells.
Some molecular and cellular analysis has been performed on ventricular regeneration and it is clear that the epicardium and the endocardium are involved.
An important factor in regeneration is neuregulin, this signling molecule is expressed in the epicard and can induce proliferation in the myocard.
In general it is thought there may be a balance between regeneration and scarring, in zebrafish regeneration is the main process.However is regeneration cannot occur as for instance in an mps1 mutant, these fish will show scarring rather that regeneration just like humans.
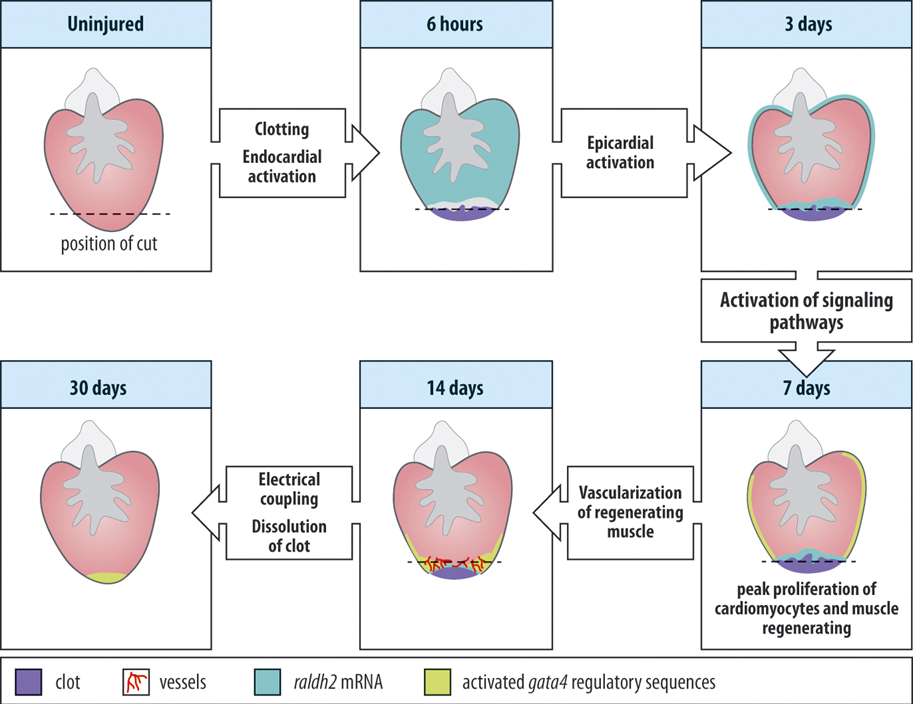
So how does the regeneration actually occur?
The uninjured heart can be seen at the upper left with the position of a cut shown by the dashed line, after resection of the heart it will bleed perfusely but soon a blood clot will form preventing the circulation from coming to a complete standstill.
Very soon after injury the endocard will be activated and start expressing certain genes, Raldh2 for instance.
In addition the epicardium will become activated, expand rapidly and start to cover the wound. This covering of the wound actually looks quite like what happens after limb amputations in newt. The epicard will signal to the muscle below causing dedifferentiation. Newly forming muscle expresses FGF and epicardial cells respond to this signal by delaminating and invading the regenerate and reform blood vessel that will help to restore a functional ventricle. The growing muscl will lead to dissolution of the clot and after about 30 days the process will be complete.
Recently it was reported that the epicard expresses an EGF like factor, Neuregulin, which can stimulate cardiomyocyte cell division. Remarkably also in uninjured zebrafish hearts, Neureglin can drive cardiomyocyte proliferation (hyperplasia) and result in cardiomegaly. Two clear examples are shown at the bottom of the lower right picture.
So Neuregulin is an important mitogen for cardiomyocytes, at least in the zebrafish.
Mammalian Heart regeneration
•Much interest in stem cells but what about myocardial cells?
•Neonatal mice can regenerate their hearts-loss of this ability correlates with loss of Erbb2 a Neuregulin coreceptor
•Loss of neuregulin
sensitivity??
Although there has been a lot of interest and money poured into stem cell therapy as a way to repair human hearts this has so far not been successful, what about doing it the fish way and stimulate the cardiomyocytes?
Although adult mammals cannot regenerate their heart, it is known in fact, that the neonatal mice can regenerate their heart in a similar way as zebrafish but loose this ability soon after birth.
Interestingly, a recent paper correlated this loss of regeneration in mice with reduction in erbb2 expression which is required for Neu signal transduction, it is a coreceptor for this signal. Thus one speculation is that mammal lose their ability to regenerate due to loss of responsivity to Neuregulin. Could restoration of sensitivity help regeneration?
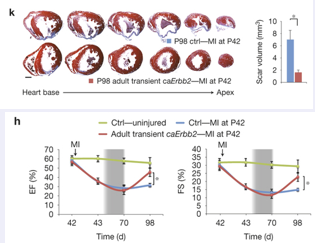
To put this to the test a trangenic mouse that had a doxyxcyline inducible- dominant active form of erbb2 was created. This transgene was then induced for some time after an infarct was created in these mice, interestingy they were able to induce cardiomyocyte proliferation a significant improvement in function, and could show a smaller scar area. As can be seen in the serial sections in K (lower line) compared to controls (upper line); see also quantification of the right.
Also a functional measurements showed an improvement (panel H). Now this is just a first experiment and will need further work, is the effect indeed due to hyperplasia?, nevertheless is suggests that if stem-cells won’t work in heart regeneration perhaps heart muscle cells can be tricked into regenerating the damaged tissue.