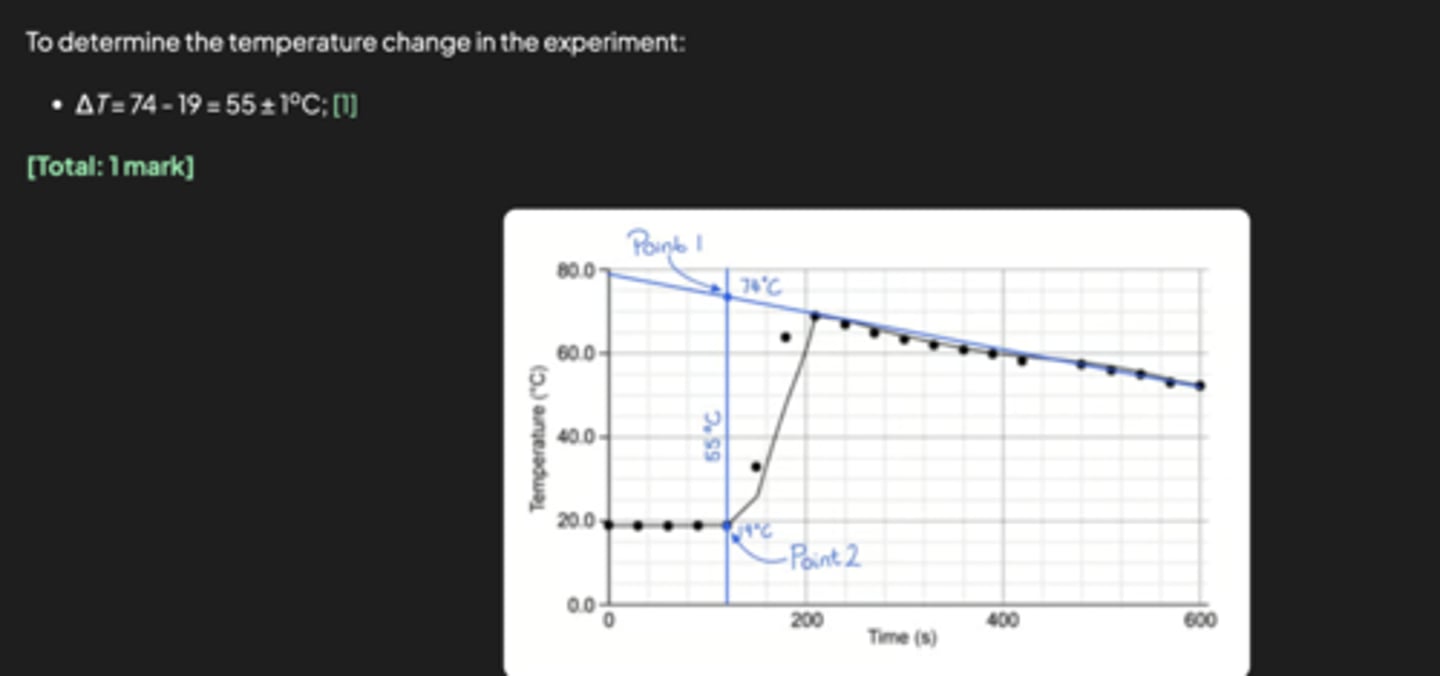
CHEM IB HL
1/231
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
232 Terms
Lattice enthalpy
Standard enthalpy change forming 1 mole of gaseous ions from solid lattice, always endothermic
Hlattice = x+y+z - Hformation
Enthalpy of atomisation
Standard enthalpy change forming 1 mole of separate gaseous atoms, always endothermic
Standard enthalpy change of solution
Enthalpy change when 1 mole of ionic substance dissolves in water to form infinitely dilute solution, can be exo/endothermic
Standard enthalpy change of hydration
Enthalpy change when 1 mole of specified gaseous ion dissolves in water to form infinitely dilute solution, always exothermic
Solvation
Process similar to hydration, but with a solvent other than water
Endothermic reactions
Energy absorbed into system from surroundings, positive enthalpy
Exothermic reactions
Energy released from system into surroundings, negative enthalpy
Entropy
Measure of system disorder, increases when system becomes more disordered and energetically stable
Thermal decomposition
Decomposition reaction where entropy of the system increases, e.g., CaCO3(s) → CaO(s) + CO2(g)
Bond forming
Process releasing energy, exothermic
Bond breaking
Process requiring energy, endothermic
Average bond enthalpy
Energy needed to break one mole of bonds in a gaseous molecule averaged over similar compounds, endothermic process
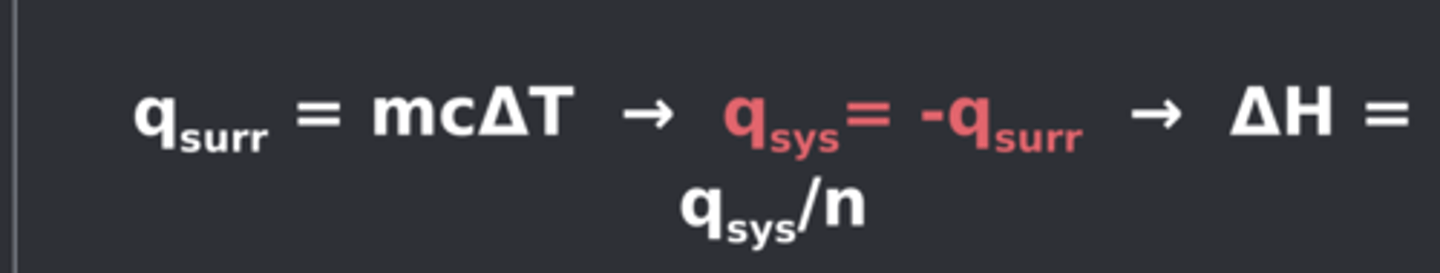
Enthalpy from surroundings
Heat transfer equation, q surr = mcT -> H = qsys/n
average bond enthalpy

Ozone reacts with:
Lower energy UV-B rays (longer wavelength)
O2 reacts with:
Higher energy UV-C light (shorter wavelength)
What UV rays primarily reach earths surface
UV-A
enthalpy change
difference in energy between reactants and products per mole of a chemical rxn
Hess' law
total enthalpy change in a chemical rxn is independent of route taken as long as initial and final conditions are the same
standard heat of formation
the change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states at 25 degrees celsius and 100 kpa

standard heat of combustion
energy released when a hydrocarbon is reacted w/ o2 to produce water and CO2.

5 ways that enthalpy change can be determined
1) calorimetry experiments
2) hess' law & enthalpy changes of known reactions
3) heats of formation (products-reactants)
4) heats of combustion (reactants-products)
5) average bond enthalpies (least accurate bc averaged over similar compounds) (bonds broken - bonds formed)
heat vs temp
heat = transfer of thermal energy
temp = measure of avg kinetic energy of molecules in a substance
open system
closed system
isolated system
open -> matter and energy can be exchanged
closed -> only energy can be exchanged
isolated -> neither exchanged
enthalpy
The total chemical energy inside a substance (heat content)
To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard conditions
These standard conditions are:
A pressure of 100 kPaA concentration of 1 mol dm-3 for all solutions. Each substance involved in the reaction is in its standard state (solid, gas or liquid). a temperature of 298 K (25 oC) is usually given as the specified temperature
The heat (or enthalpy) of neutralization
change in enthalpy that occurs when one mole of H20 is formed under standard conditions with reactants and products in standard states. happens when an acid and alkali are reacted. exothermic
formation, combustion, and neutralization enthalpies -> exo or endothermic?
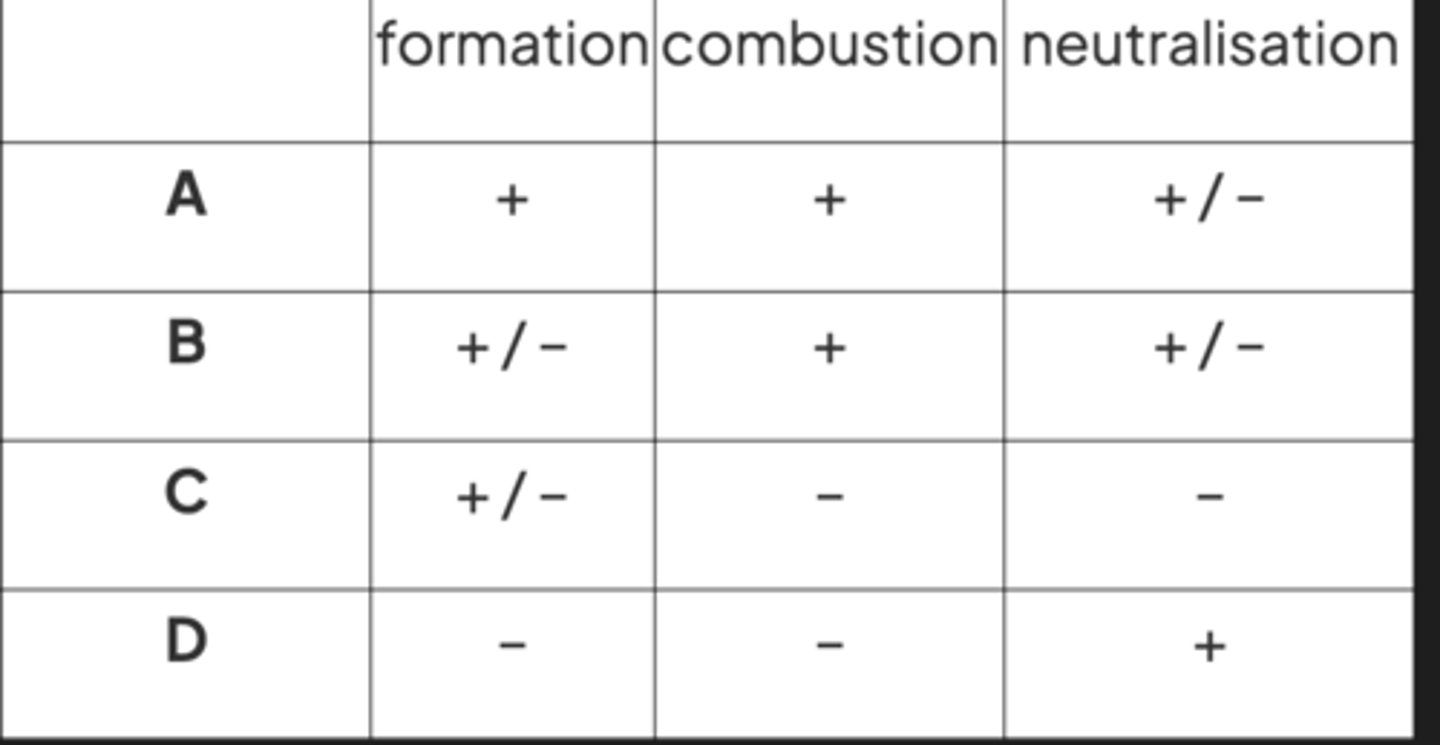
image q
A (D incorrect bc One of the reactants is used in excess to ensure a complete reaction occurs)

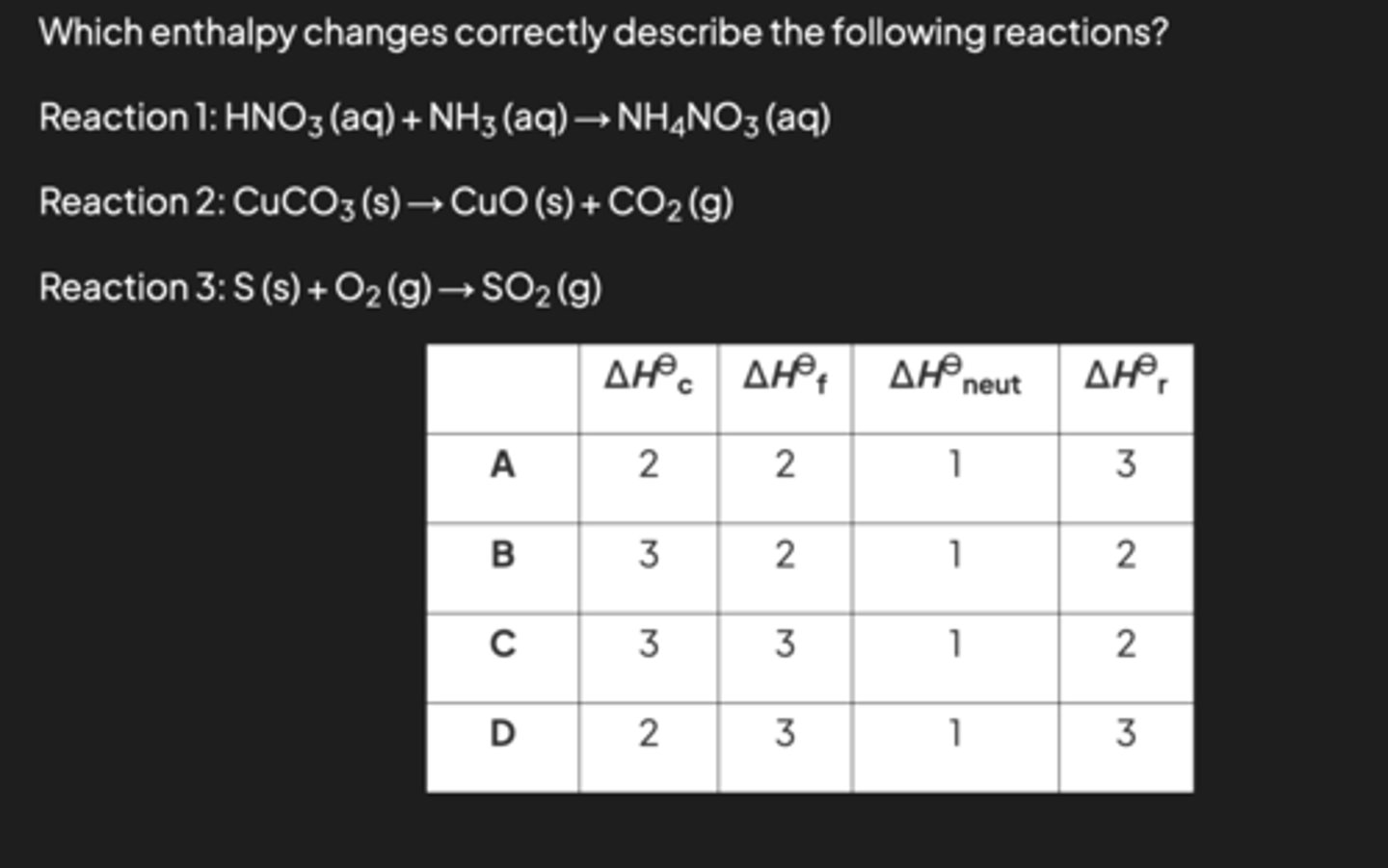
image q
C because reaction 2 is not combustion because there is no reaction with oxygen and it is not formation because it starts from a compound. Reaction 3 is the combustion of sulfur AND the formation of sulfur dioxide.
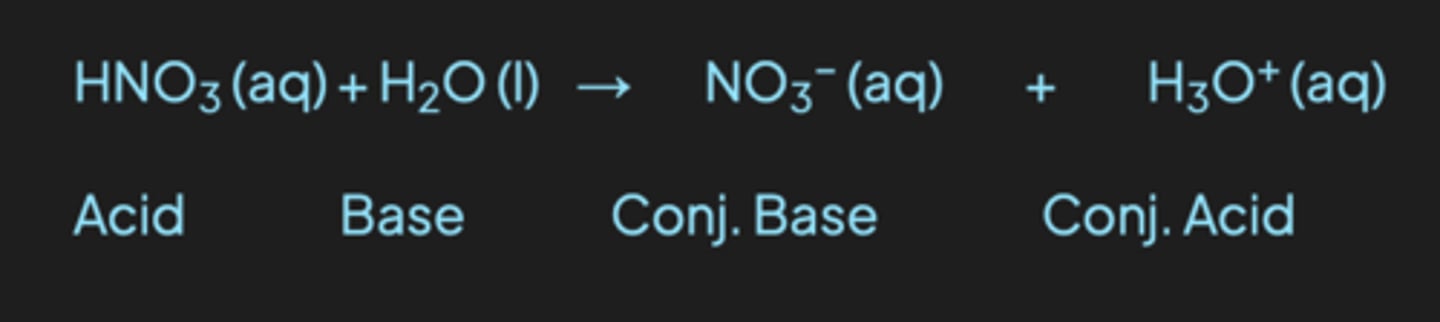
The Bronsted-Lowery definition
refers to the loss or gain of an H+ (proton). The acid is a proton donor, and the base is a proton acceptor
An equation to demonstrate how nitric acid can behave as a Brønsted-Lowry acid when it reacts with water is:
% error
experimental value-accepted value/accepted value x 100
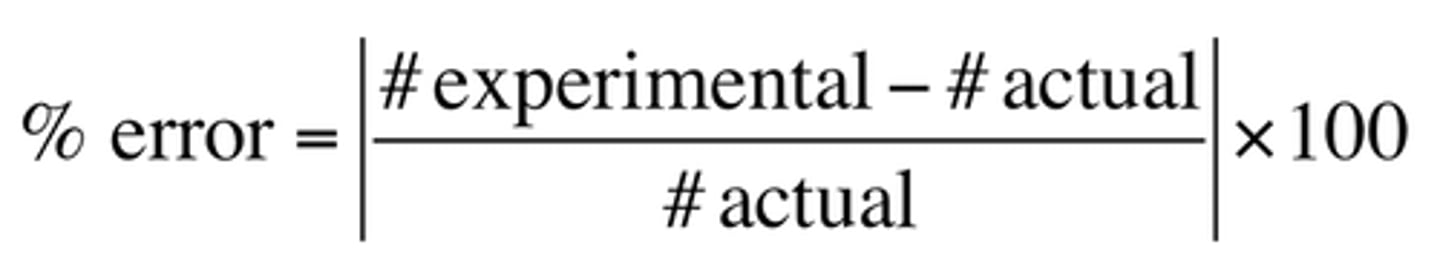
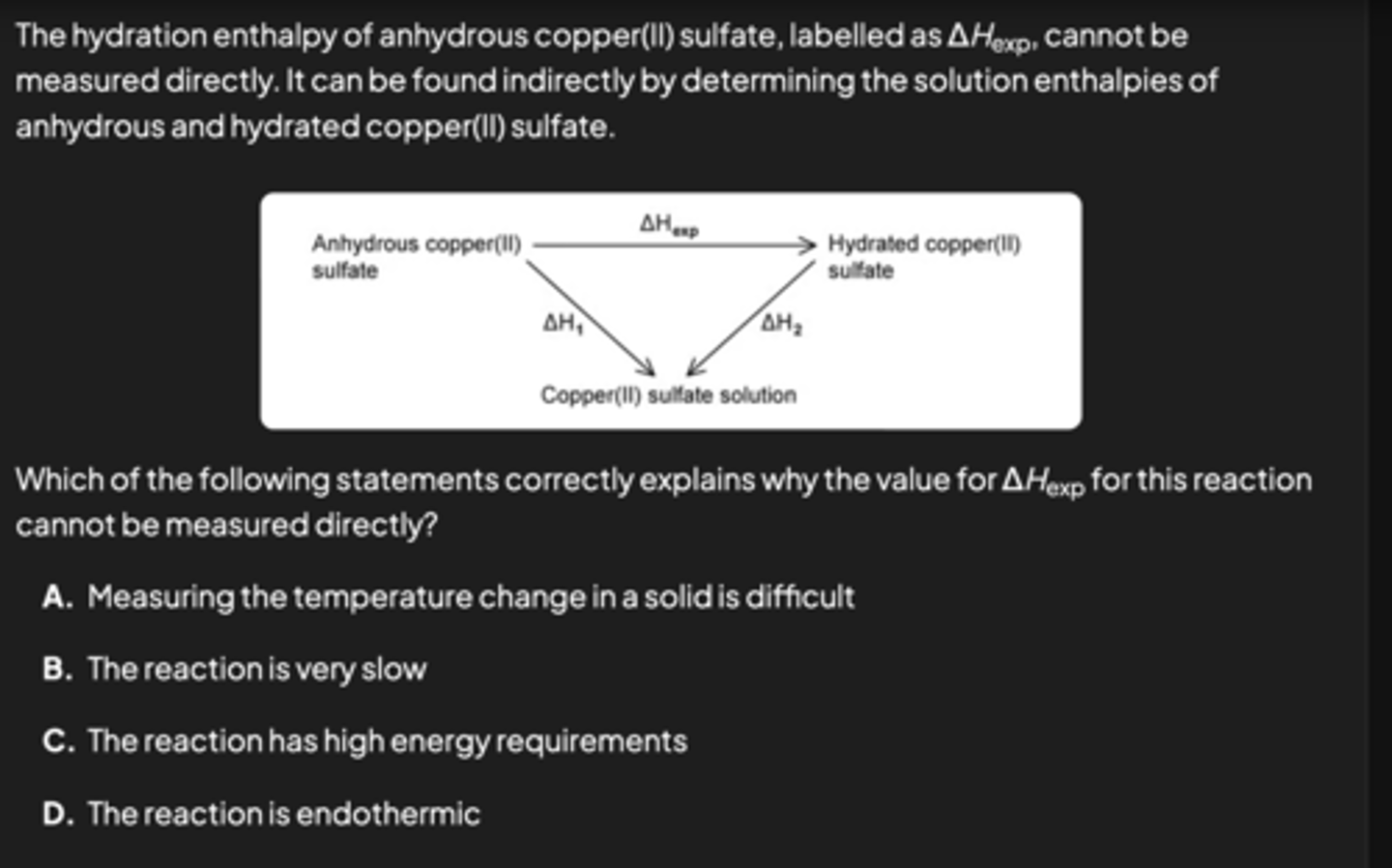
D

Index of hydrogen deficiency
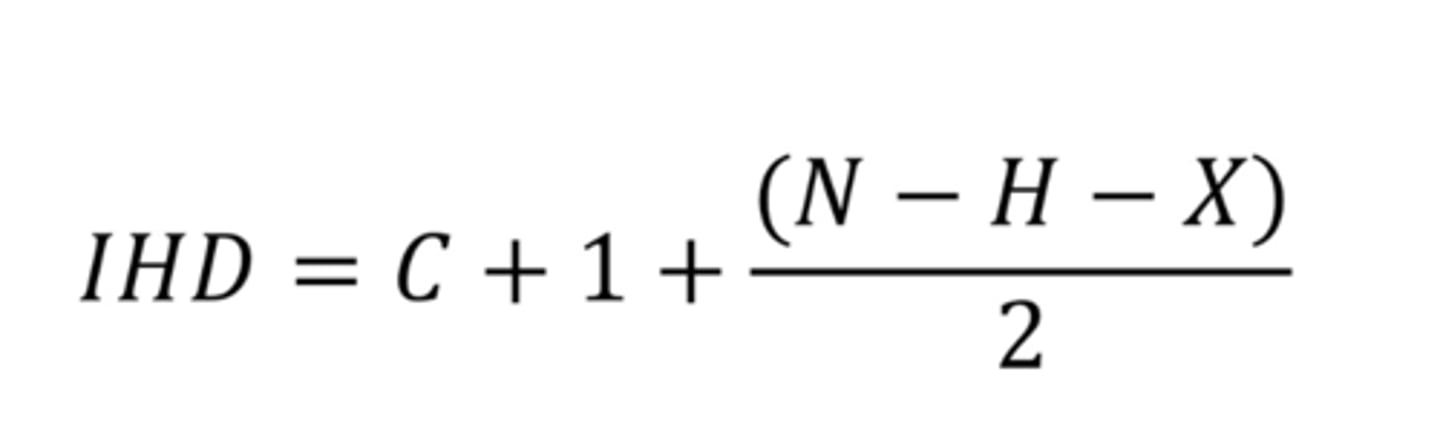
why loss of ozone is an international environmental concern
a.Loss of ozone allows UV radiation to reach Earth's atmosphere
UV light can cause cancer / tissue damage to humans
how CFCs destroy ozone
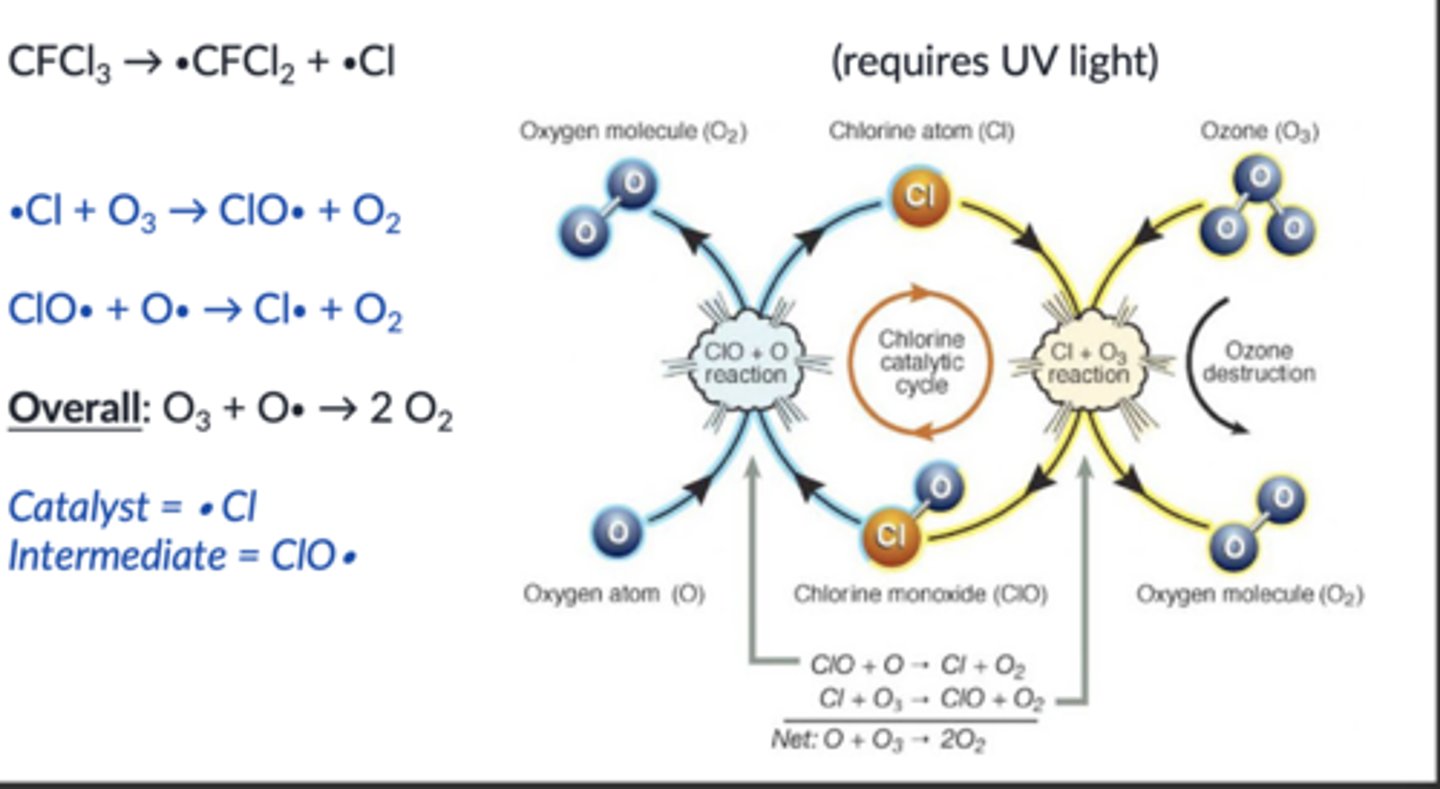
how NOx destroys ozone

bond breaking and making (exo vs endo)

The deduction that can be made from ozone absorbing UV radiation in the region of 340 nm and molecular oxygen absorbing it in the region of 242 nm is:
242 nm is a shorter wavelength than 340 nm. Shorter wavelength corresponds to higher energy;
The bond in oxygen must be stronger than the bond in ozone because it takes more energy to break the bond.
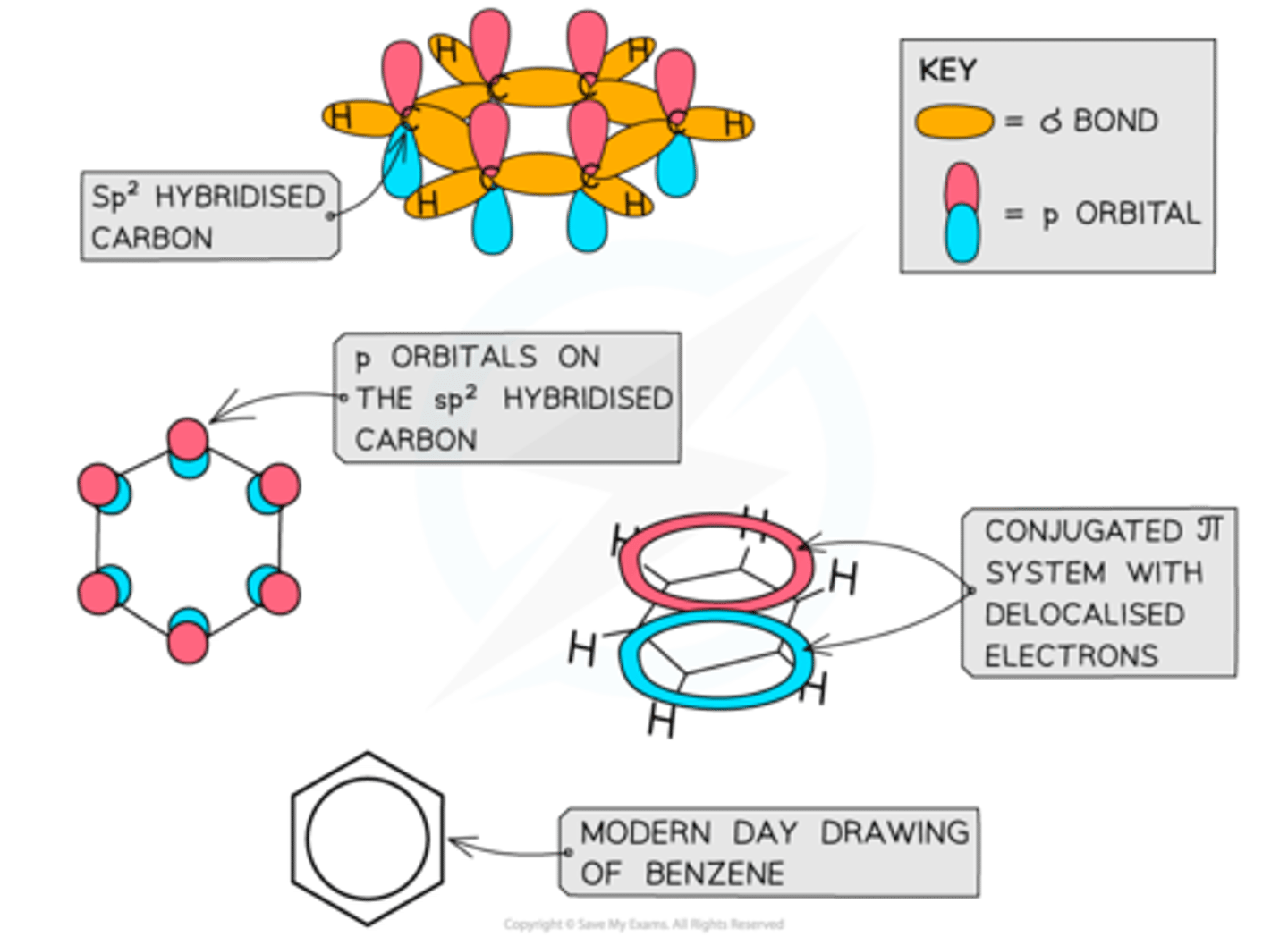
A delocalized π bond is:
a bond in which the electrons are free to move over more than two nuclei.
ozone reacts with:
lower energy UV-B
O2 reacts with:
higher energy UV-C
stability and enthalpy relationship
more stable means lower enthalpy
In nucleophilic substitution reactions involving halogenoalkanes...
the halogen atom is replaced by a nucleophile
strength of nucleophile
The hydroxide ion, OH-, is a stronger nucleophile than water because it has a full negative chargeThis means that it has a readily available lone pair of electrons
what halogenoalkanes undergo SN1 vs SN2
Tertiary halogenoalkanes undergo SN1 reactions
Secondary halogenoalkanes undergo a mixture of both SN1 and SN2 reactions depending on their structure
Primary halogenoalkanes undergo SN2 reactions. for SN2 reactions (one step) there must be room to attack
Protic solvents
solvents contain a hydrogen atom bonded to a very electronegative nitrogen or oxygen atom
ex ammonia, ethanol, water
aprotic solvents
contain hydrogen atoms but they are not bonded to an electronegative atom
This means that they cannot participate in hydrogen bonding. ex ethyl ethanoate, propanone
SN1 reactions are best conducted using protic, polar solvents
SN2 reactions are best conducted using aprotic, non-polar solvents
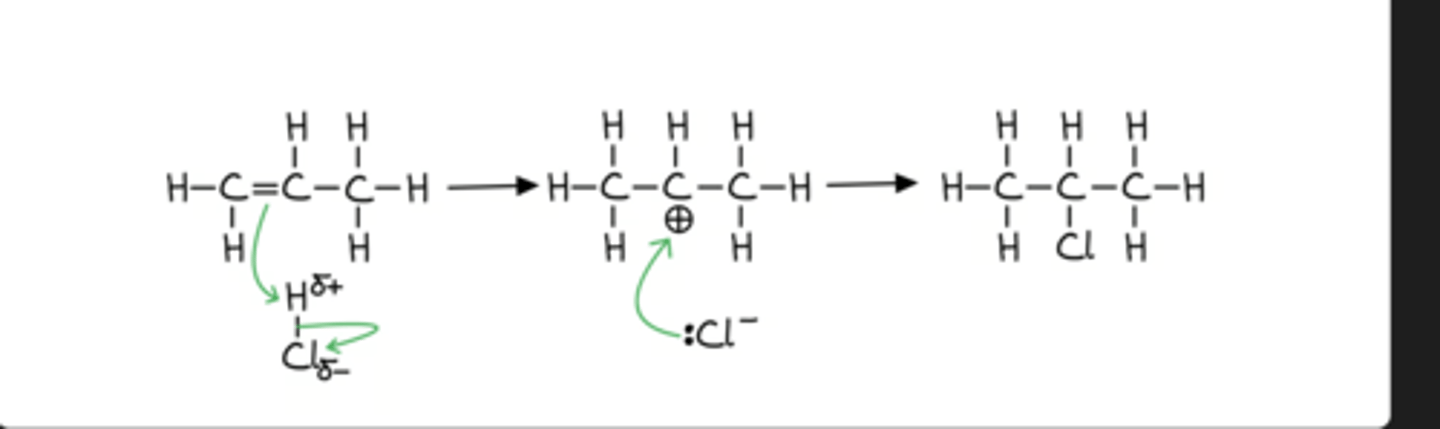
For electrophilic addition mechanisms, the curly arrows must:
Start from a lone pair of electrons or an area of high electron density, e.g. the C=C bond
Move towards a δ+ electrophile or the positive charge of a carbocation
Interhalogens
compounds that contain two or more different type of halogens
The electrophilic addition reactions of alkenes with hydrogen halides, halogens and interhalogens are the same. The difference is whether the electrophile is due to a permanent or temporary dipole
In an electrophilic addition reaction of an interhalogen to an alkene, the most electronegative halogen ends up bonded to the most substituted carbon atom
The stability of the carbocation intermediate is as follows:
tertiary > secondary > primary
More R (carbon containing) groups stabilize the carbocation.
When more than one carbocation can be formed, the major product of the reaction will be the one that results from the nucleophilic attack of the most stable carbocation.
conjugated pi systems benzene
Conjugated π systems arise from alternating double and single bonds in which the electrons are delocalised
Like other aromatic compounds, benzene has a planar structure due to the sp2 hybridisation of carbon atoms and the conjugated π system in the ring
electrophile
Electron pair acceptor in an organic mechanism. Attracted to areas with a lot of electrons/high negative charge.
nucleophile
electron pair donator
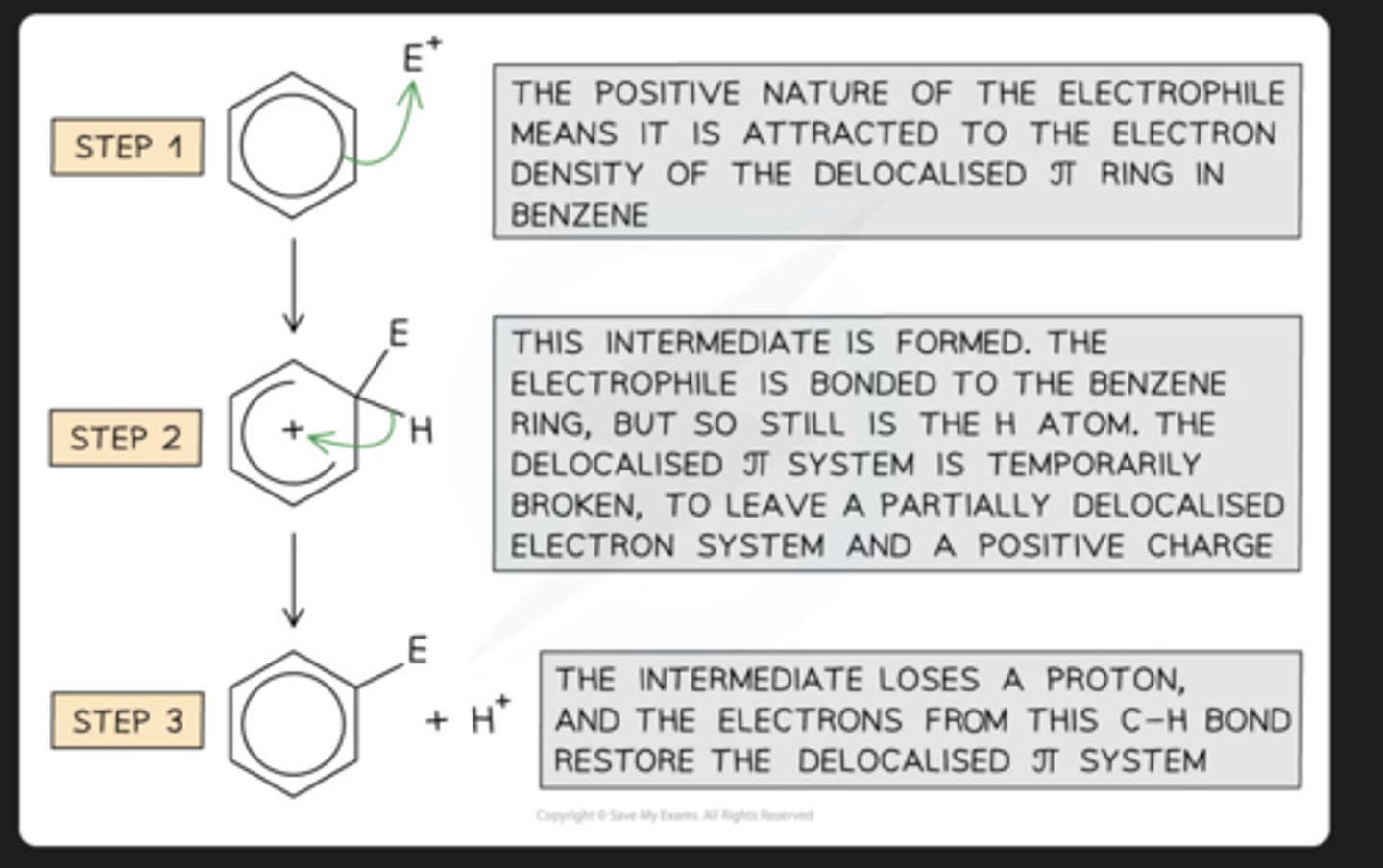
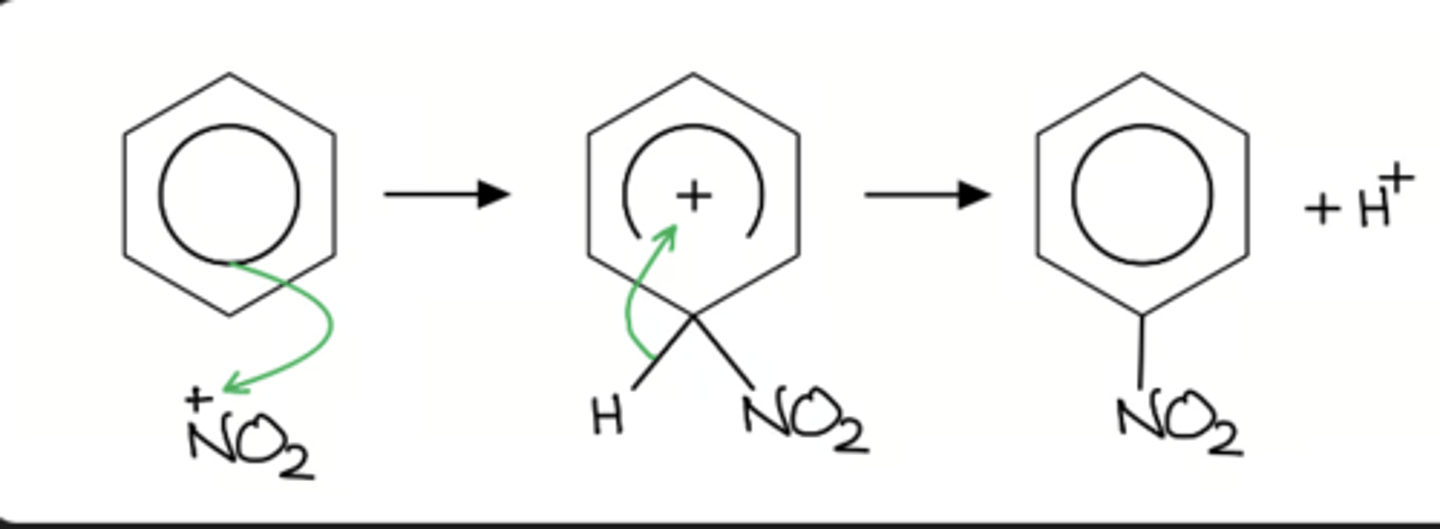
General Electrophilic Substitution Mechanism:
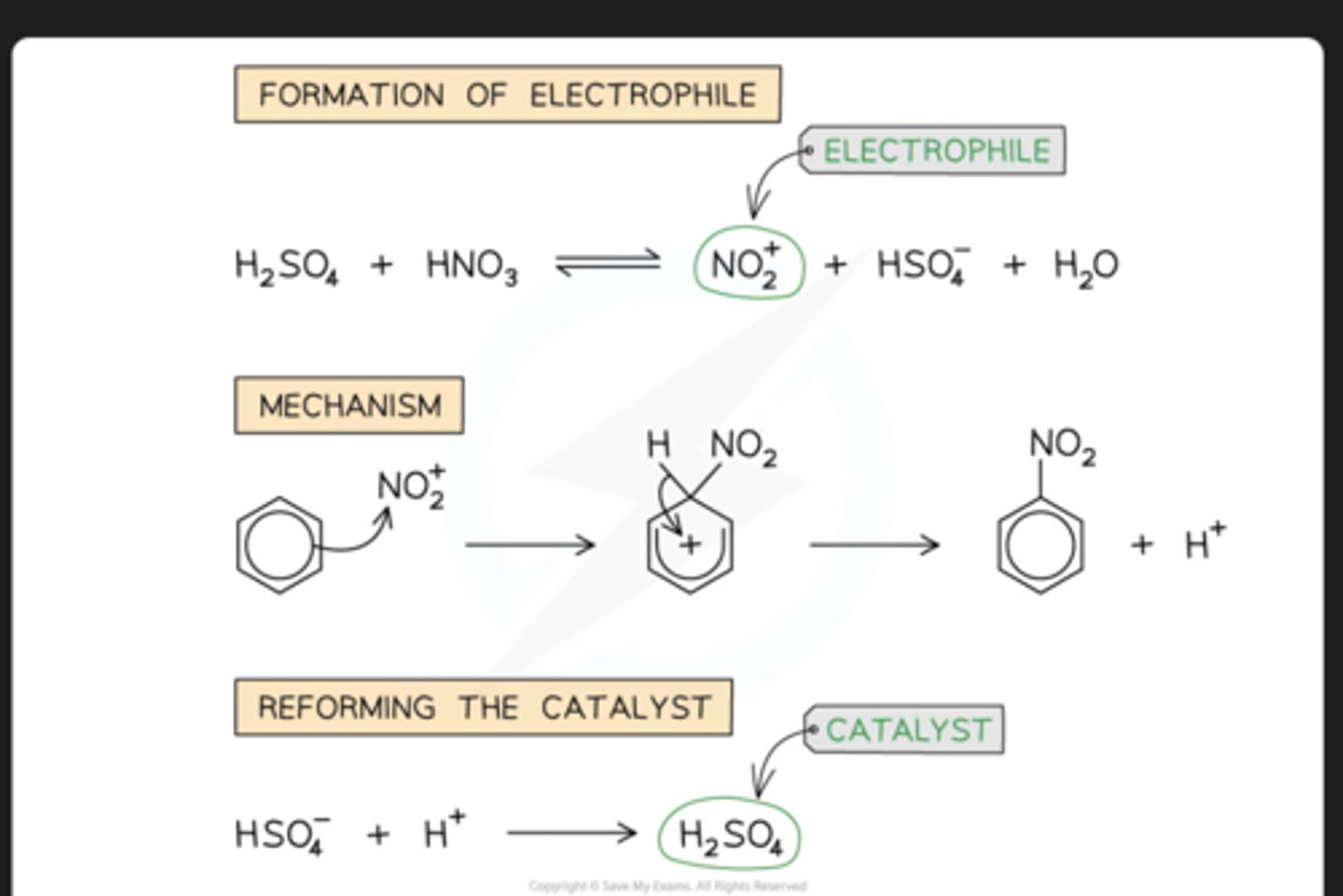
nitration of benzene steps
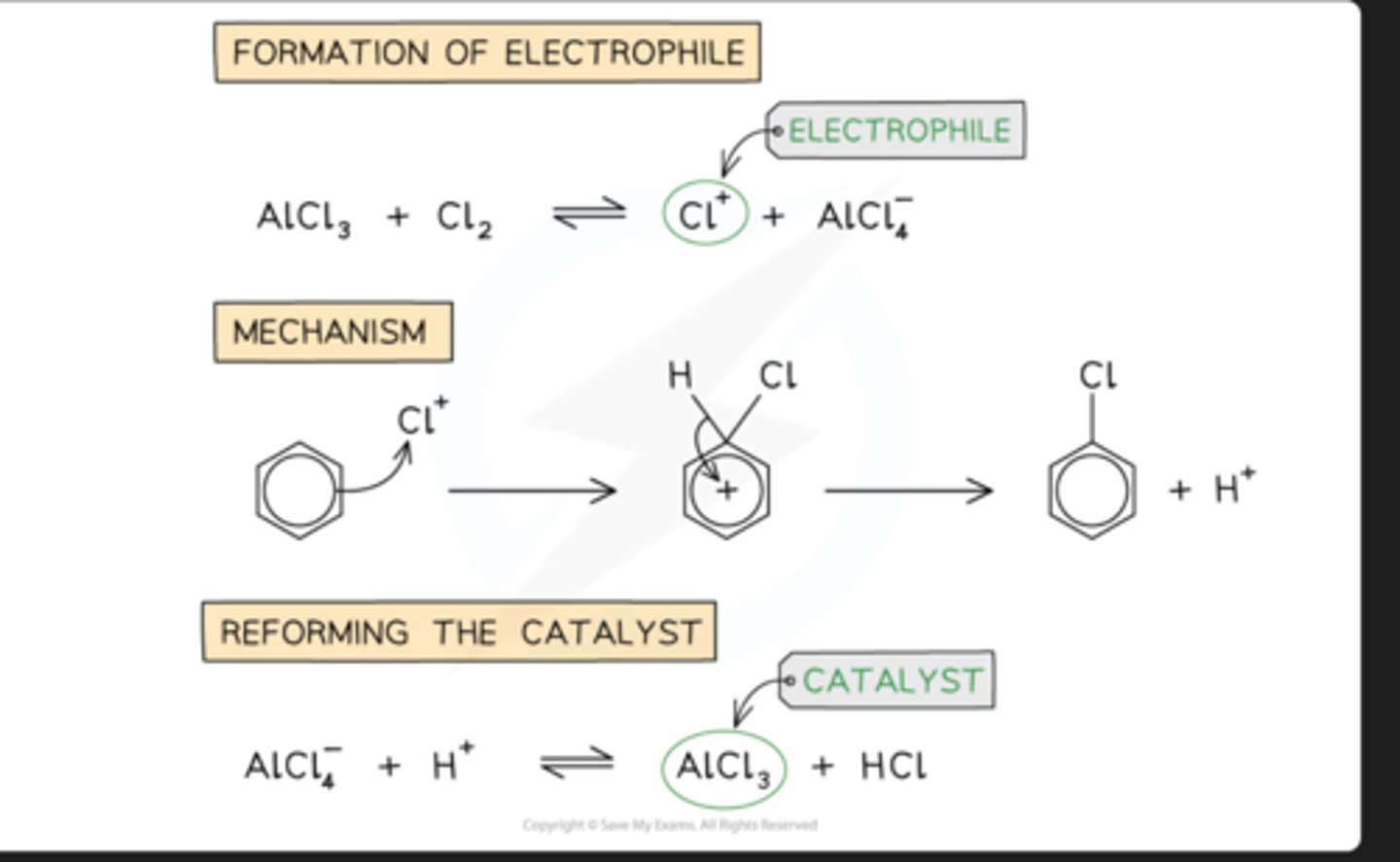
chlorination of benzene steps
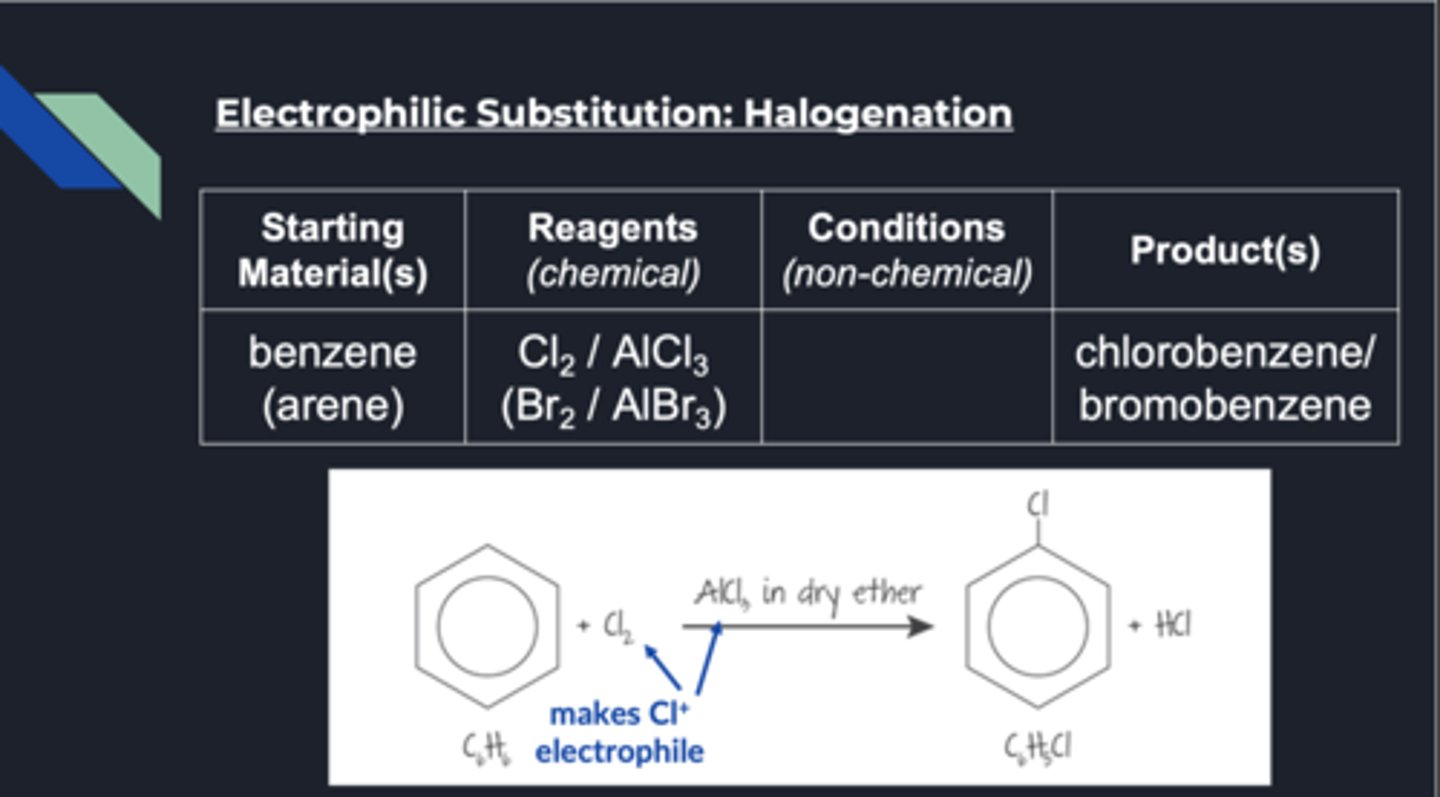
halogenation benzene conditions
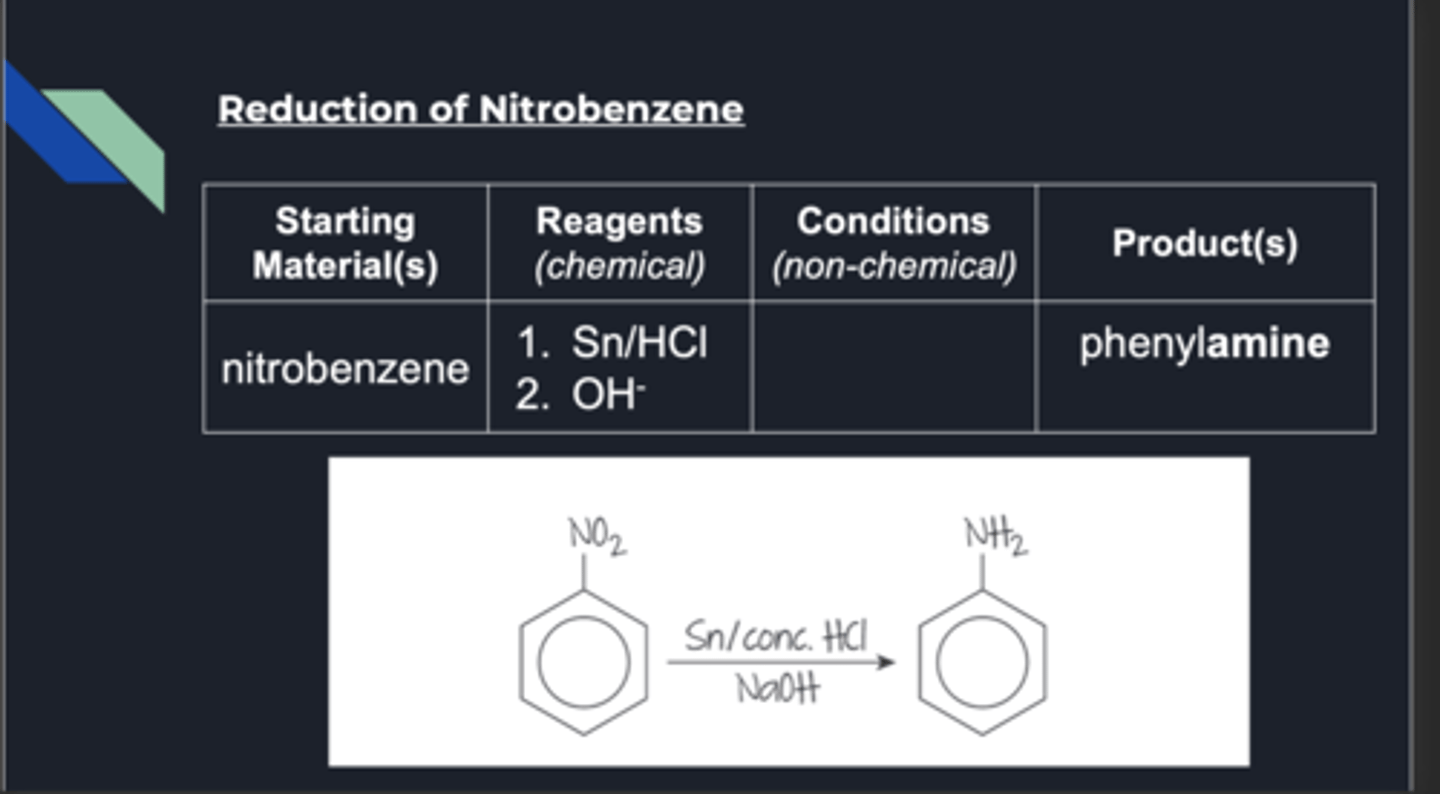
reduction of nitrobenzene
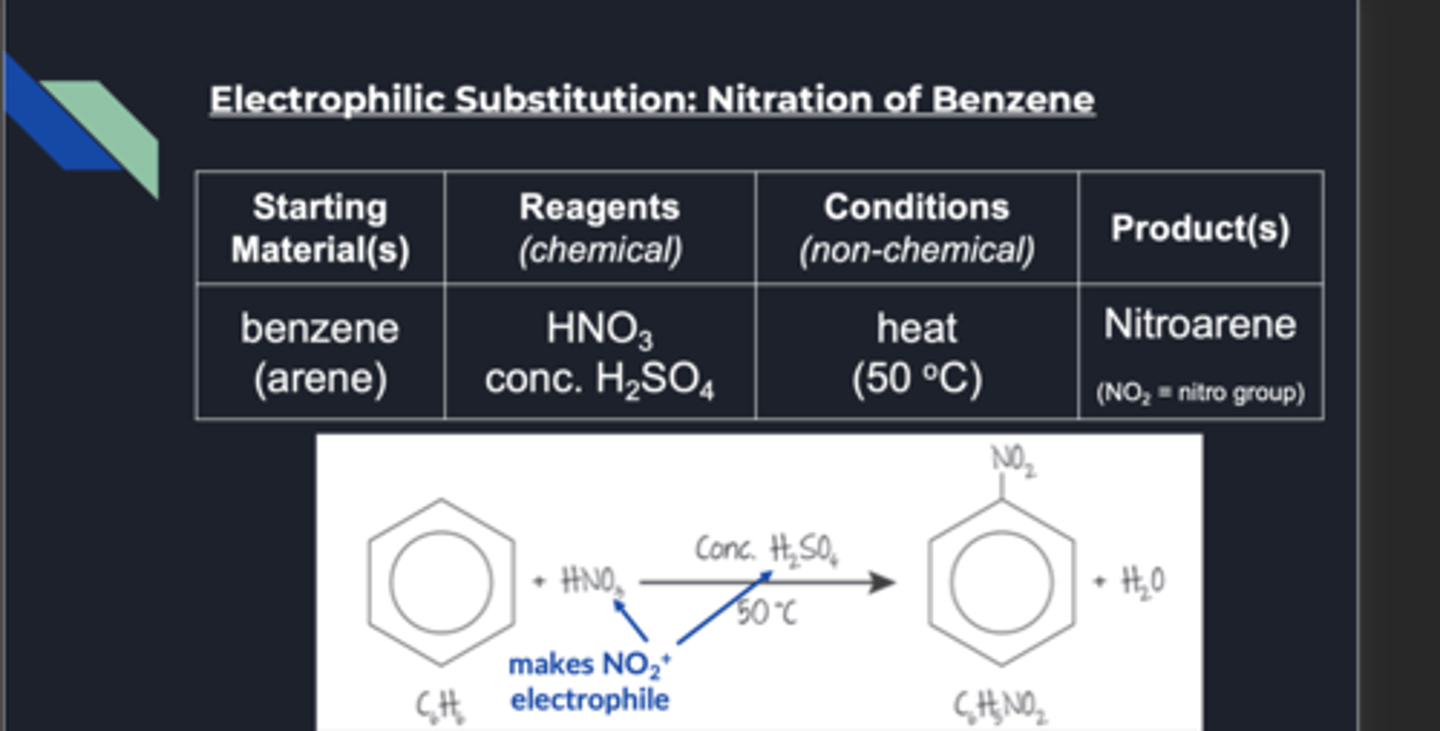
nitration of benzene
backside attack
nucleophile attacks opposite to the side of the leaving group
why rxn conditions of halogenations are different for alkanes and benzene
benzene has delocalized pi bonds that are susceptible to electrophilic attack, and alkanes do not
SN2 rate of rxn depends on..
conc of halogenoalkane & nucleophile
SN1 rate of rxn depends on..
conc of halogenoalkane
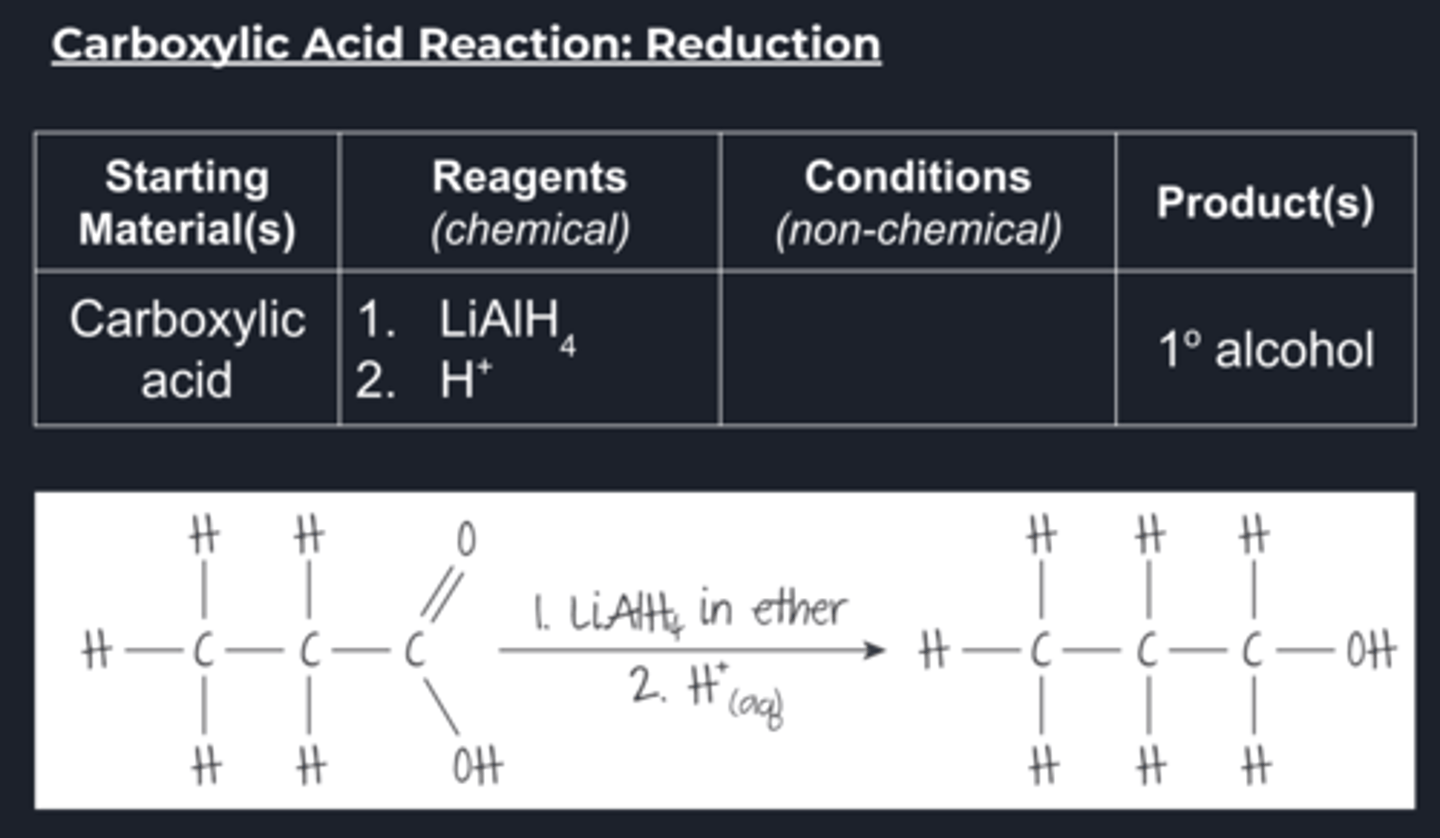
carboxylic acid reduction conditions
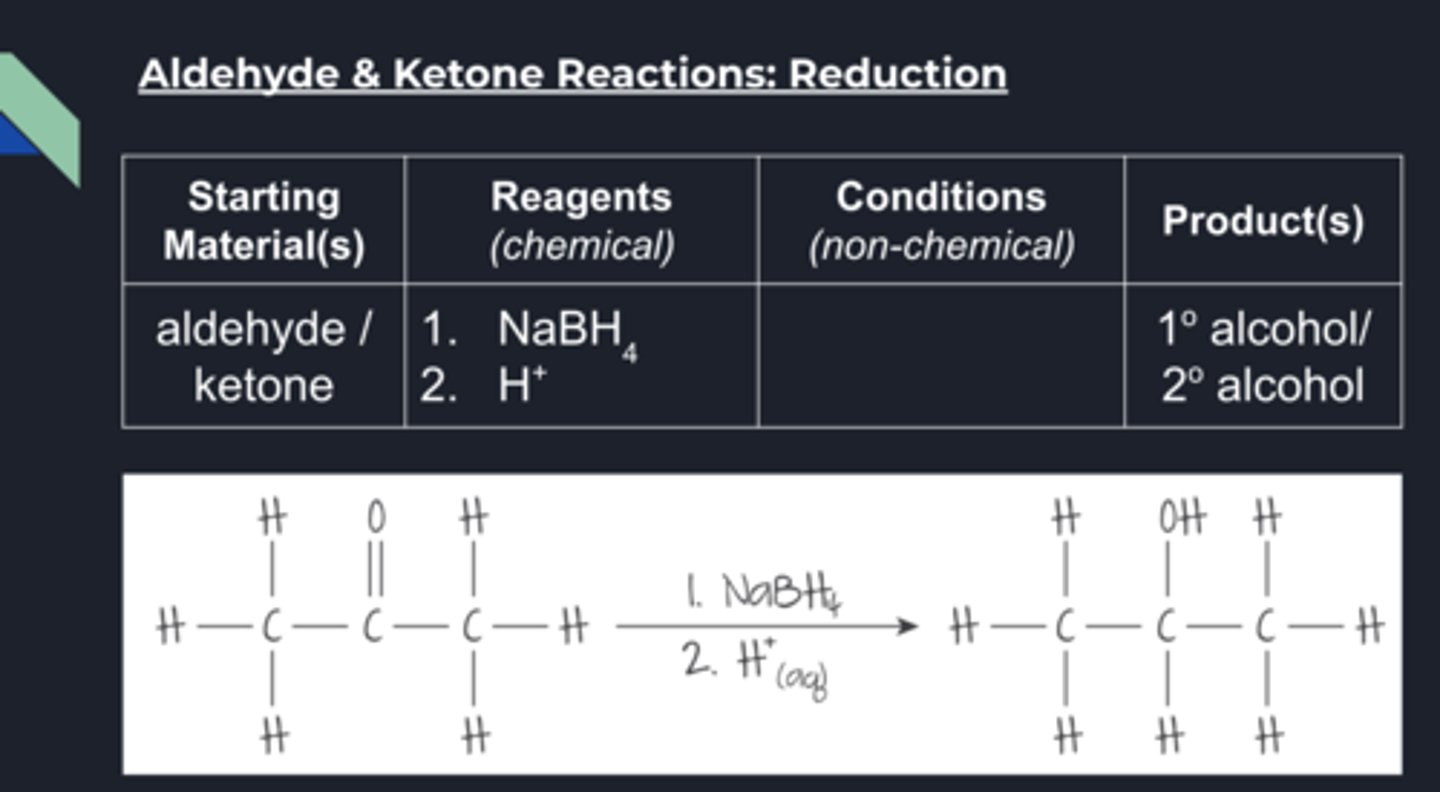
reduction of aldehyde & ketones conditions
lewis acid and lewis base
A Lewis acid is therefore any substance, such as the H+ ion, that can accept a pair of nonbonding electrons. In other words, a Lewis acid is an electron-pair acceptor. A Lewis base is any substance, such as the OH- ion, that can donate a pair of nonbonding electrons.
sn1 bromobutane and naoh
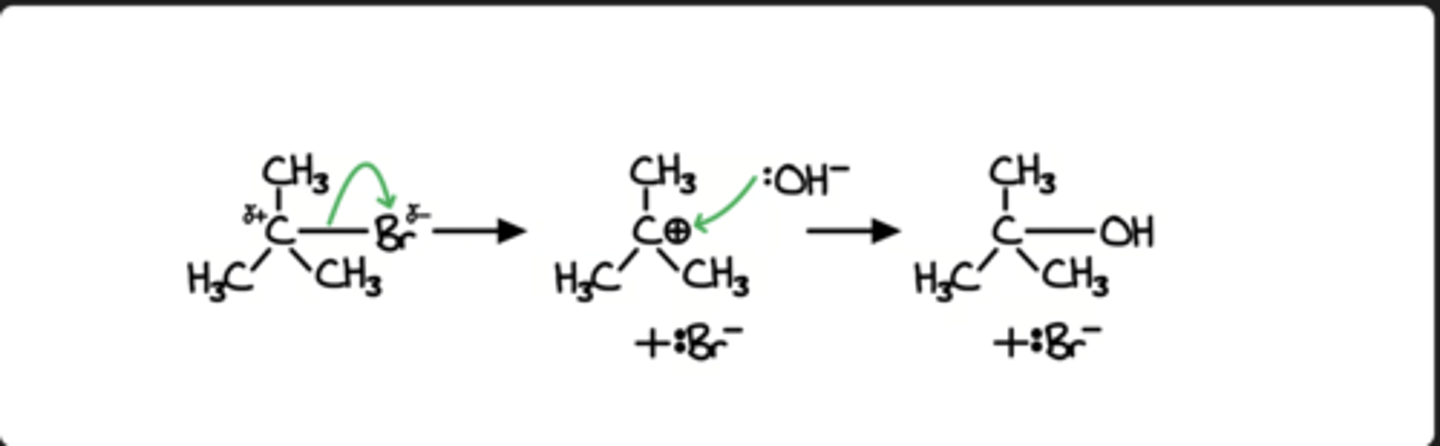
Propene to chloropropane
secondary carbocation is more stable than primary carbocation therefore this is the major product.
remember, The major product has more alkyl groups stabilising the carbocation intermediate
The Kekulé structure of benzene is incorrect because:
All benzene carbon-carbon bond lengths are equal / between the lengths of carbon-carbon single and double bonds; [1]
All benzene carbon-carbon bond strengths are equal; [1]
Benzene is a regular hexagon / planar structure; [1]
All benzene carbon-carbon bonds have a bond order of 1.5; [1]
Benzene (preferentially) undergoes substitution reaction (not addition); [1]
Benzene is more stable than expected ; [1]
Benzene does not react with bromine water; [1]
1,2-Disubstitution reactions of benzene only forms one isomer; [1]
The equation for the formation of the nitronium ion is:
H2SO4 + HNO3 → HSO4- + NO2+ + H2O

nitration of benzene
aniline is also known as...
phenylamine (what nitrobenzene reduces to)
A halogenoalkane reactant that can be used to slowly produce butan-1-ol by reacting with aqueous sodium hydroxide is:
1-chlorobutaneOR1-fluorobutane; [1]
The key word in this question is slowly
Stronger carbon-halogen bonds mean that the rate of reaction is slowerTherefore, fluorobutane is the slowest halogenoalkane to react and iodobutane is the fastest to react
bromoethane reacting with aqueous alkali when heated to form ethanol
The halogen is replaced by a nucleophile, OH-
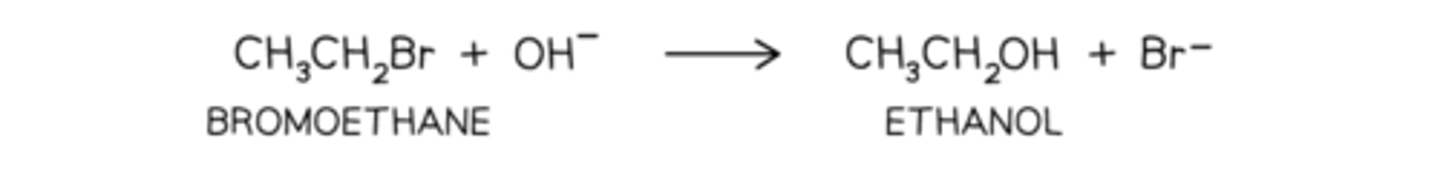
A nucleophile
is an the electron rich species that will react with an electron poor species · A substitution implies that one group replaces another.
homolytic and heterolytic fission
The breaking of bond in which one electron is transferred to each fragment is called homolytic fission. The unequal breaking of the covalent bond resulting in the formation of anions and cations is called heterolytic fission.
Heterolytic fission (or heterolysis) - the pair of electrons is taken by one of the atoms. Homolytic fission (or homolysis) - the pair of electrons is split between the separated atoms.
steric bulk
The amount of space that a group of atoms takes is called the "steric bulk". An example of steric effects is steric hindrance. This is when a large group in a molecule makes reactions not work.
ex from photo
The bromine atom of the bromoethane molecule causes steric hindrance
This means that the hydroxide ion nucleophile can only attack from the opposite side of the C-Br bond
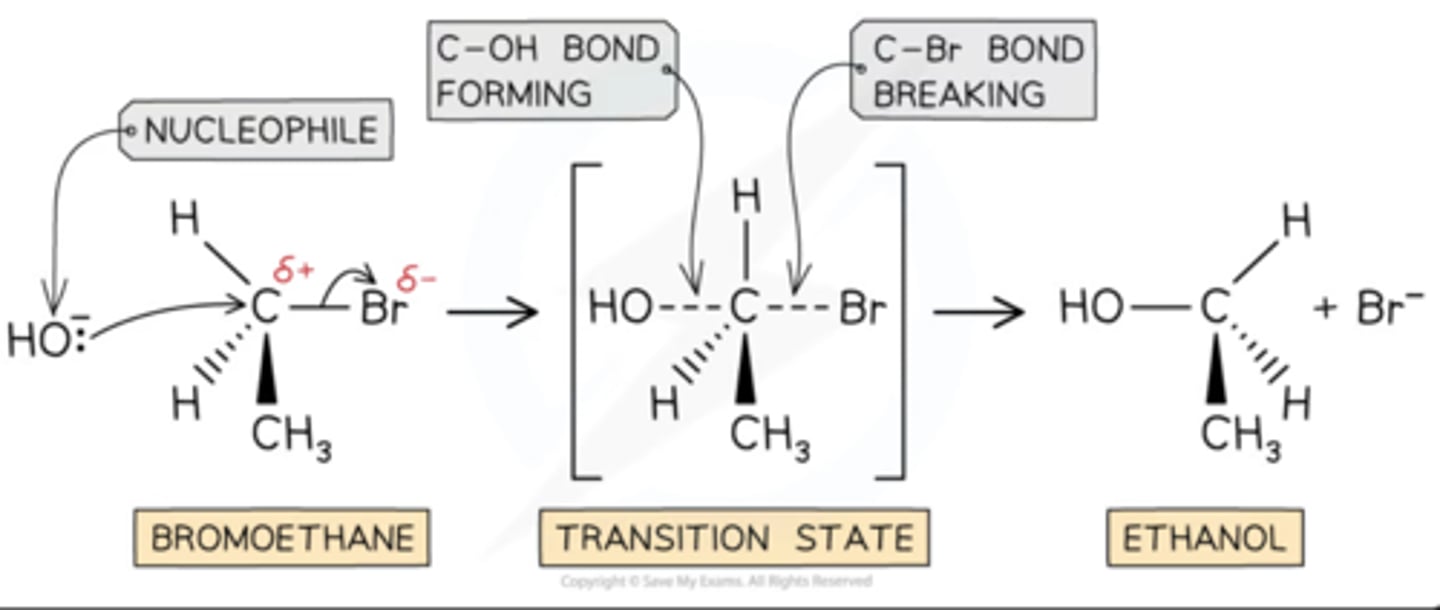
need to know: the carbon center of the electrophile that is attacked by the nucleophile is always inverted during an SN2 reaction
-inversion of the chiral carbon via backside attack
not the case with sn1, Since the nucleophile attacks the carbocation only after the leaving group has departed, there is no need for back-side attack. The carbocation and its substituents are all in the same plane, meaning that the nucleophile can attack from either side.
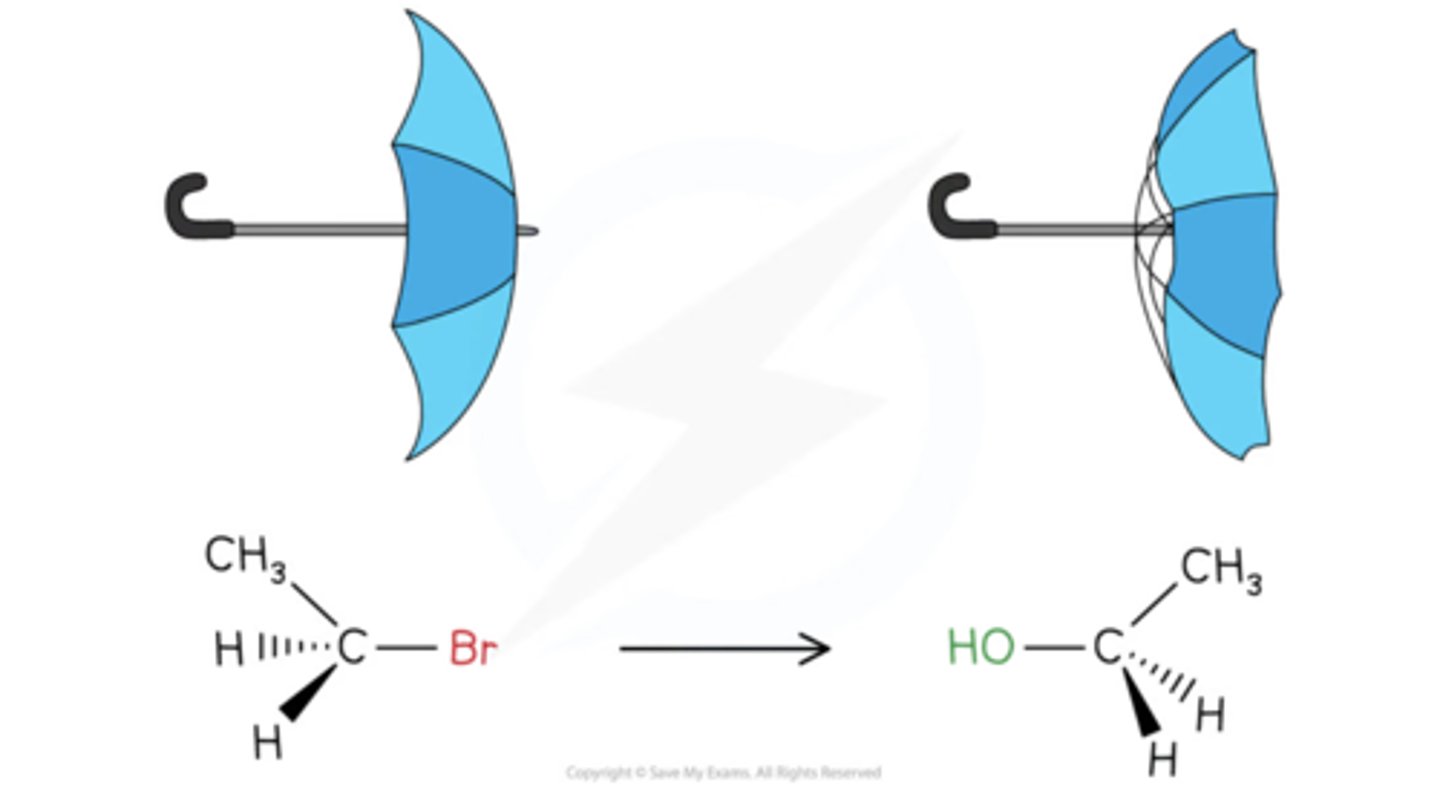
electronegativity and nucleophile relationship
Electronegativity is the tendency of an atom to attract shared pairs of electrons. Thus, the ability to donate electrons pairs decreases with increasing electronegativity. Thus, the lesser the electronegativity better would be a nucleophile.
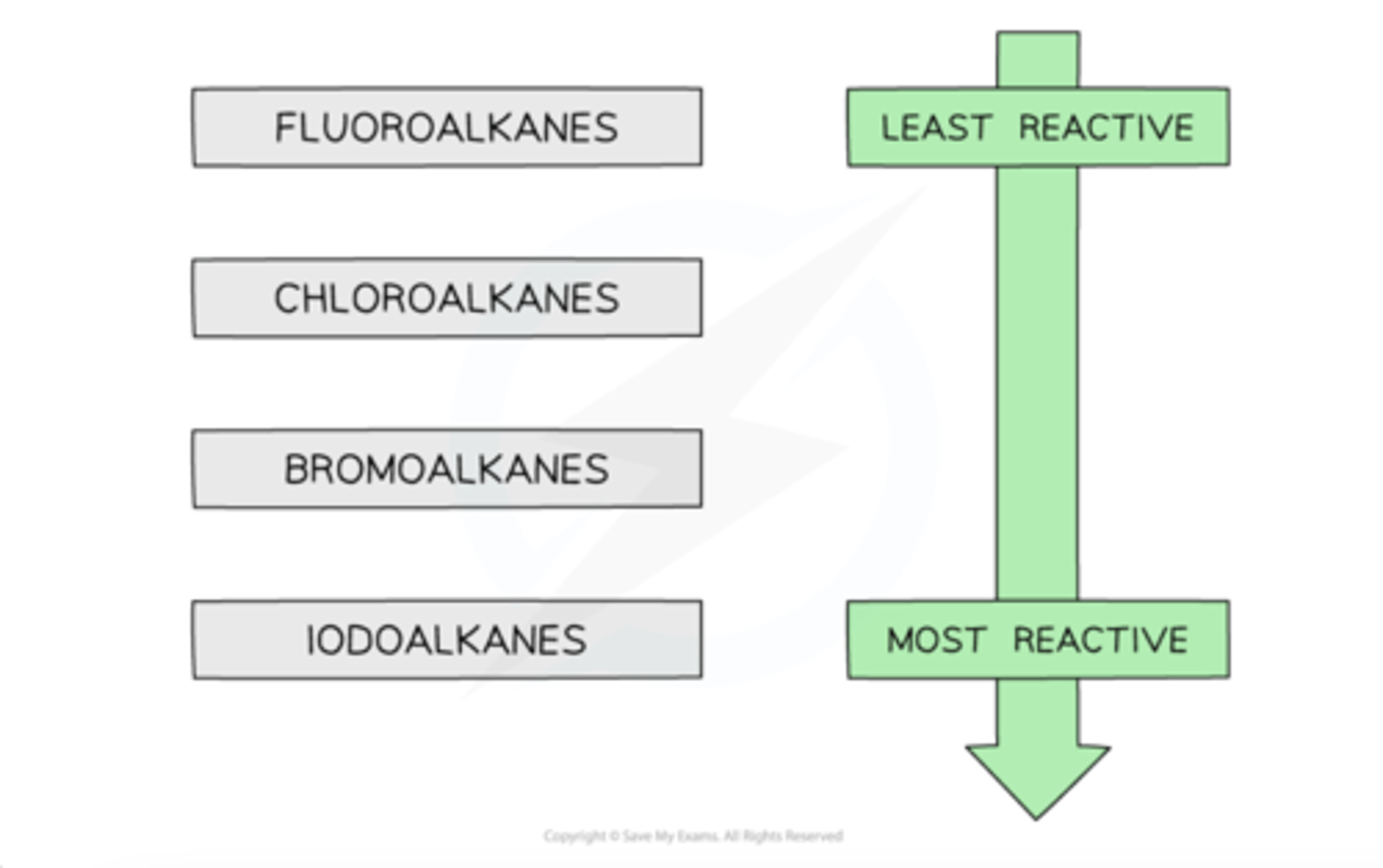
C-I vs C-F which is most likely to undergo substitutition rxn
C-I bond requires the least energy to break, and is therefore the weakest carbon-halogen bond. During substitution reactions, the C-I bond will breaks heterolytically as follows:
R3C-I + OH- → R3C-OH + I-
The C-F bond, on the other hand, requires the most energy to break and is, therefore, the strongest carbon-halogen bondFluoroalkanes will therefore be less likely to undergo substitution reactions
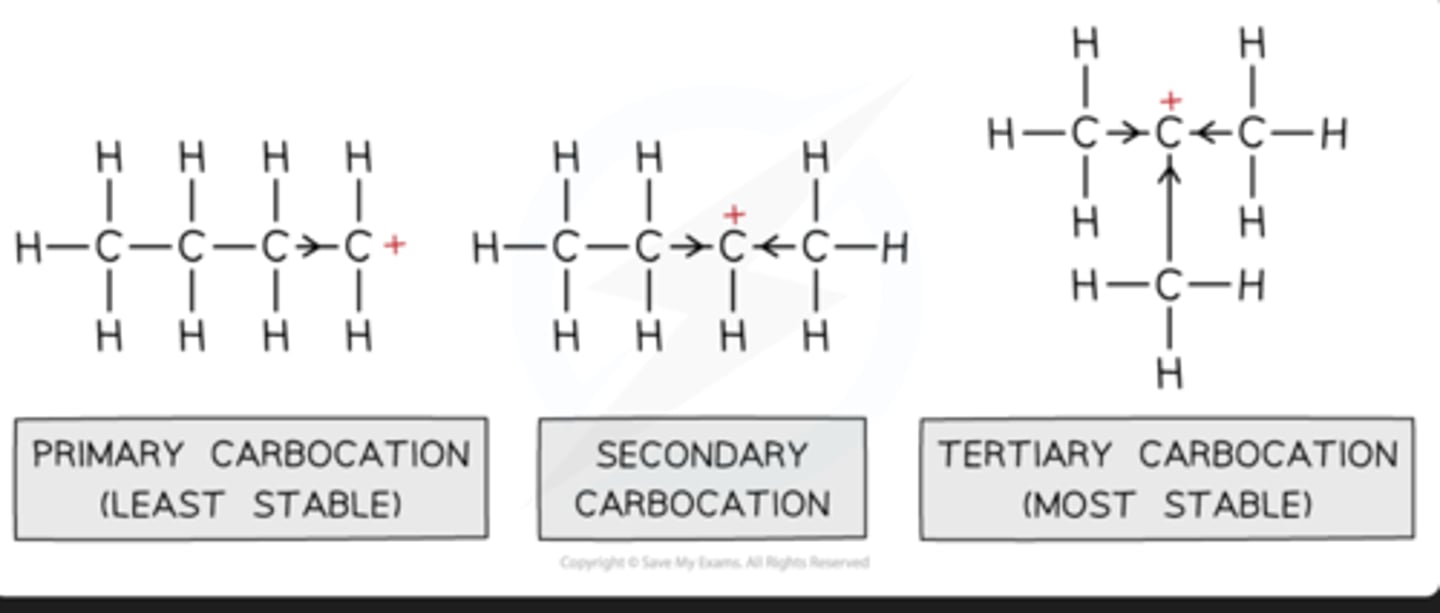
tertiary, secondary, primary halogenoalkanes and sn1 & sn2 reactions
Tertiary halogenoalkanes undergo SN1 reactions, forming stable tertiary carbocations
Secondary halogenoalkanes undergo a mixture of both SN1 and SN2 reactions depending on their structure
Primary halogenoalkanes undergo SN2 reactions, forming the less stable primary carbocations
This has to do with the positive inductive effect of the alkyl groups attached to the carbon which is bonded to the halogen atom. The alkyl groups push electron density towards the positively charged carbon, reducing the charge density. In tertiary carbocations, there are three alkyl groups stabilising the carbocationIn primary carbocations, there is only one alkyl group. This is why tertiary carbocations are much more stable than primary ones
SN1 reactions are best conducted using protic, polar solvents
SN2 reactions are best conducted using aprotic, non-polar solvents
why?
In SN1, Protic polar solvent stabilises carbocation intermediates and halide ions.
In SN1 reactions, the rate-determining step involves the formation of a carbocation intermediate. Protic solvents, which contain hydrogen atoms capable of forming hydrogen bonds, stabilize the carbocation intermediate through solvation. This stabilization lowers the energy barrier for carbocation formation and increases the rate of the reaction.
On the other hand, in SN2 reactions, the rate-determining step involves the simultaneous attack of the nucleophile and departure of the leaving group. Aprotic solvents, lacking acidic protons that can form strong hydrogen bonds, do not solvate the nucleophile as much. This lack of solvation enhances the nucleophilicity of the nucleophile, making it more available to attack the electrophilic carbon atom in the substrate. Aprotic solvents also reduce the likelihood of competitive solvation of the leaving group, favoring its departure.
electrophilic substituion usually occurs with..
aromatic compounds ex benzene
halogenation benzene conditions
reagents: cl2/alcl3 or bR2/albr3
produce chlorobenzene or bromobenzene
nitration benzene conditions
reagents: hno3, h2so4
also requires heat
produce nitroarene
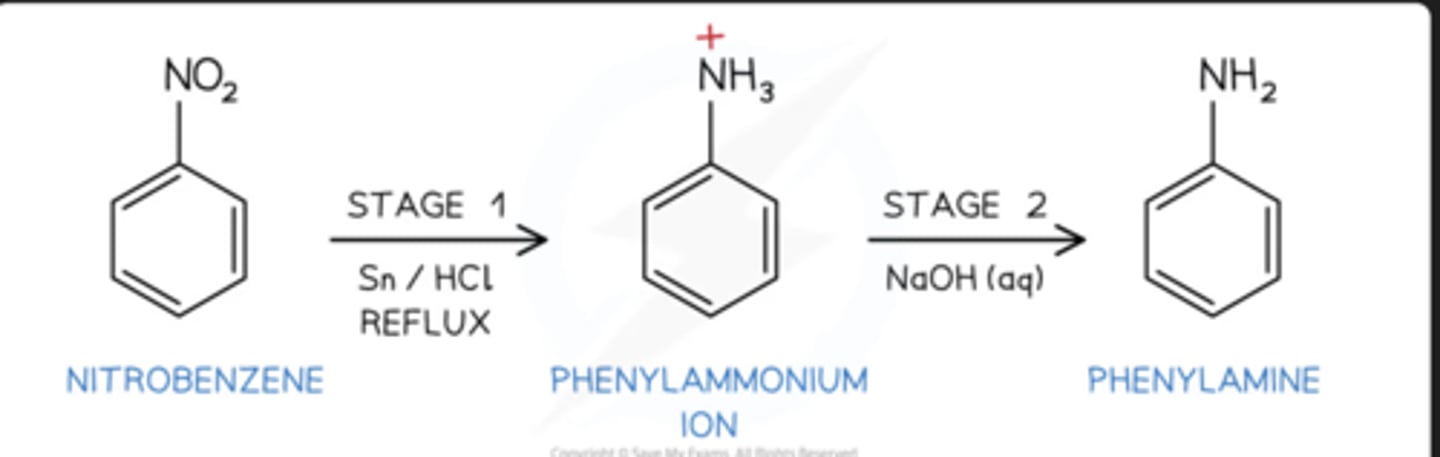
reduction benzene conditions
reagents: sn/hcl and naoh. produce phenylamine (aniline). do heat under reflux
The two most common reducing agents for carbonyl compounds are:
Lithium aluminium hydride, LiAlH4, in anhydrous conditions, commonly dry ether, followed by the addition of aqueous acidThis is the stronger of these reducing agents and can reduce carboxylic acids
Sodium borohydride, NaBH4, in aqueous or alcoholic solutionsThis is the less hazardous of these reducing agents but it cannot reduce carboxylic acids
Both of these reagents produce the nucleophilic hydride ion, H-
green chemistry
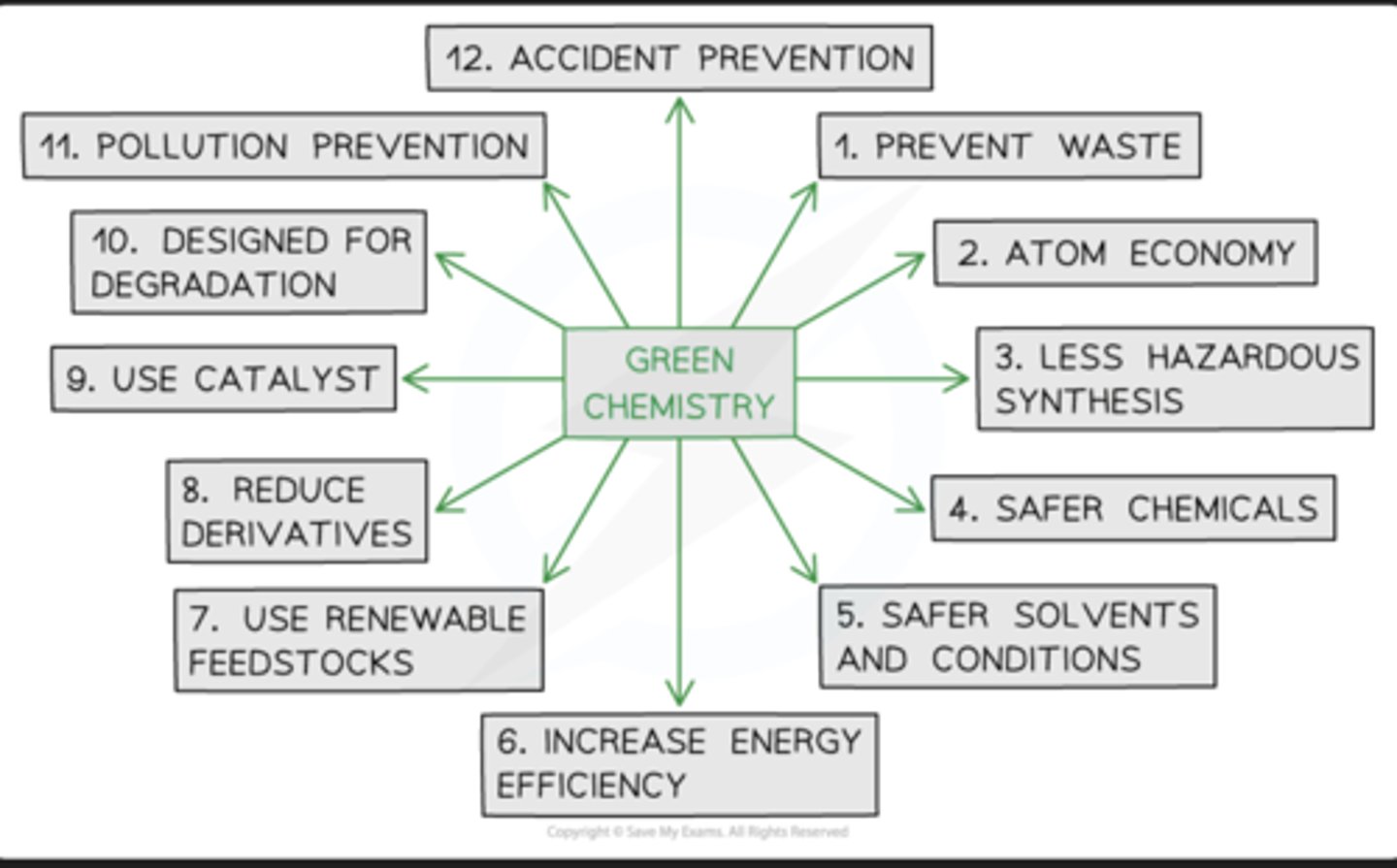
atom economy
the conversion efficiency of a chemical process in terms of all atoms involved and the desired products produced.
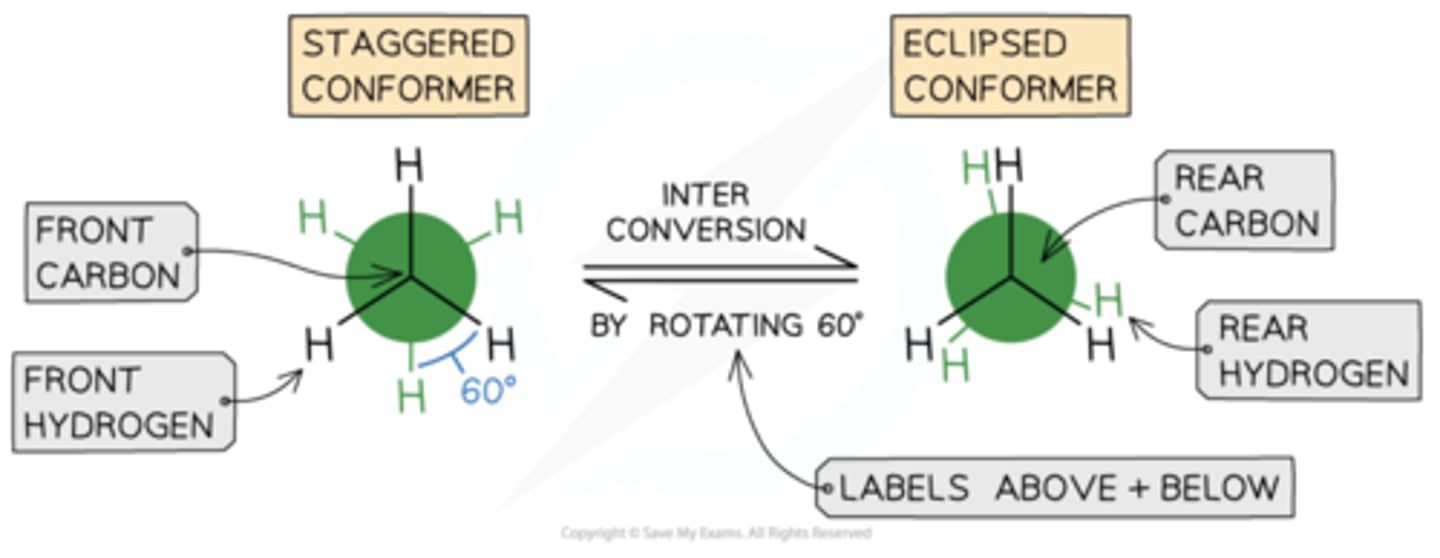
staggered vs eclipsed conformers
The staggered conformer has angles between hydrogen atoms on adjacent carbons of 60 degrees, as shownIt is also more stable / lower energy than the eclipsed conformer because the C-H bonds are as far apart as possible to minimise the repulsion between the electrons in the C-H bonds
The eclipsed conformer has angles between hydrogen atoms on adjacent carbons of 0o, this is not shown in the diagrams so that the conformation can be seenThe eclipsed conformer is less stable / higher energy due to the repulsion between the electrons in the C-H bonds that are closer together
The free rotation that causes these conformers means that it is easy to interconvert from one conformer to the other and backThis is also the reason that it is almost impossible to isolate a single conformer
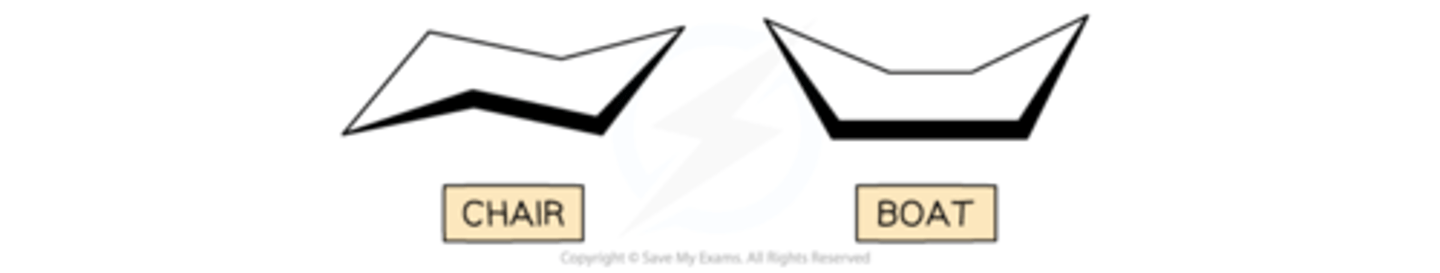
cyclohexane conformational isomerism
A common example of this is cyclohexane, C6H12Cyclohexane isomers exist in boat and chair forms:
The boat form is less stable / higher energy as there are four eclipsed bonds causing strain on the overall structureThere is also repulsion of the hydrogen atoms on the end of the boat structure
It is possible to "flip" between the boat and chair forms which explains the difficulty in isolating just one of the formsDuring the interconversions, it also possible to get other structures commonly called the half chair and the twisted boat
rotation about bonds
In saturated compounds, the atoms / functional groups attached to the single, σ-bonded carbons are not fixed in their position due to the free rotation about the C-C σ-bondThis causes conformational isomers, as previously discussed
In unsaturated compounds, the groups attached to the C=C carbons remain fixed in their positionThis is because free rotation of the bonds about the C=C bond is not possible due to the presence of a π bond
Cis / trans isomerism in cyclic compounds
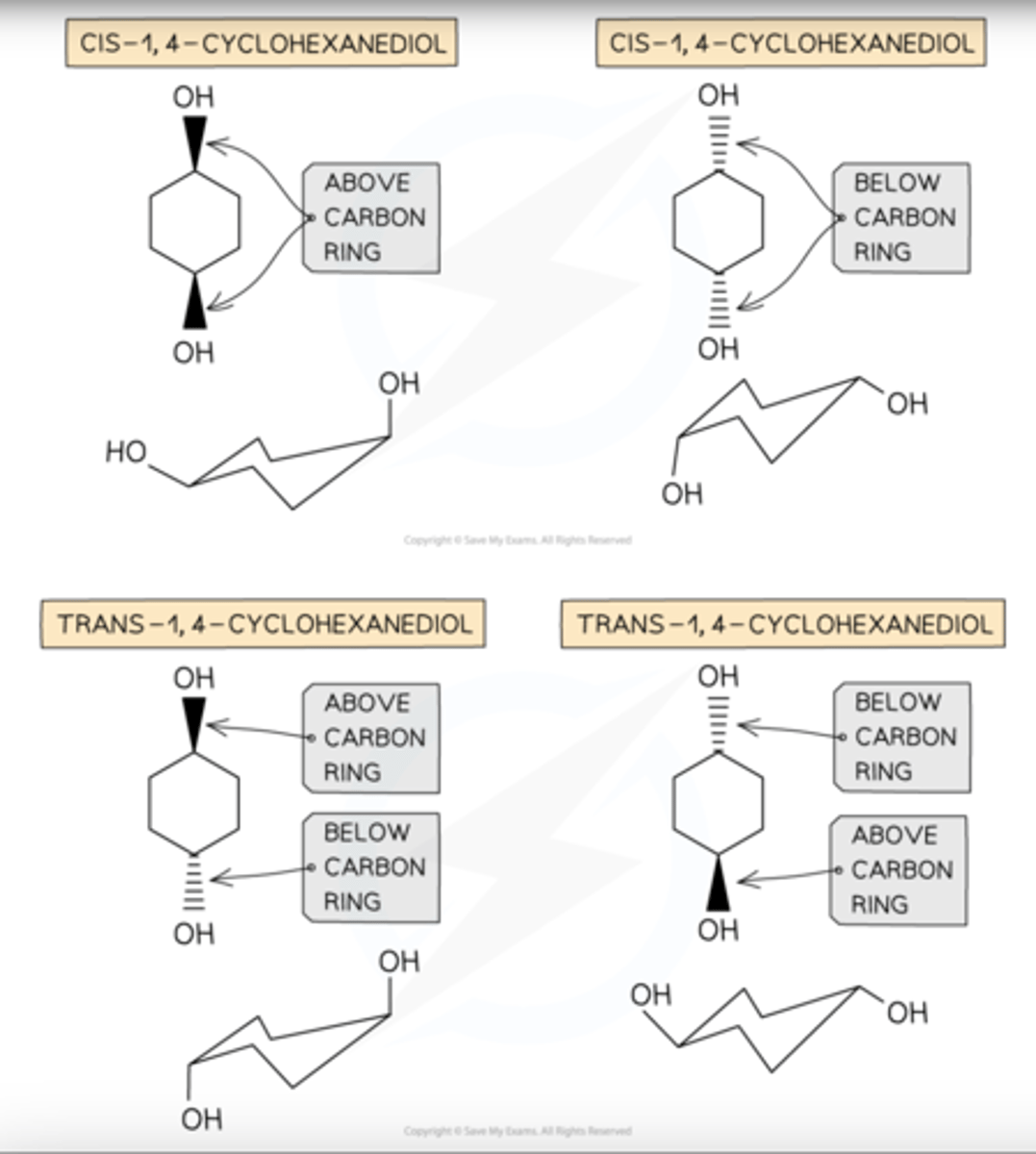
E/Z in relation to cis and trans
When they are cis, you get Z. When they are trans you get E.
EX cis-pent-2-ene or (Z) pent-2-ene
chiral centre
A carbon atom that has four different atoms or groups of atoms attached to it is called a chiral carbon or chiral centre
The carbon atom is described as being asymmetric, i.e. there is no plane of symmetry in the molecule
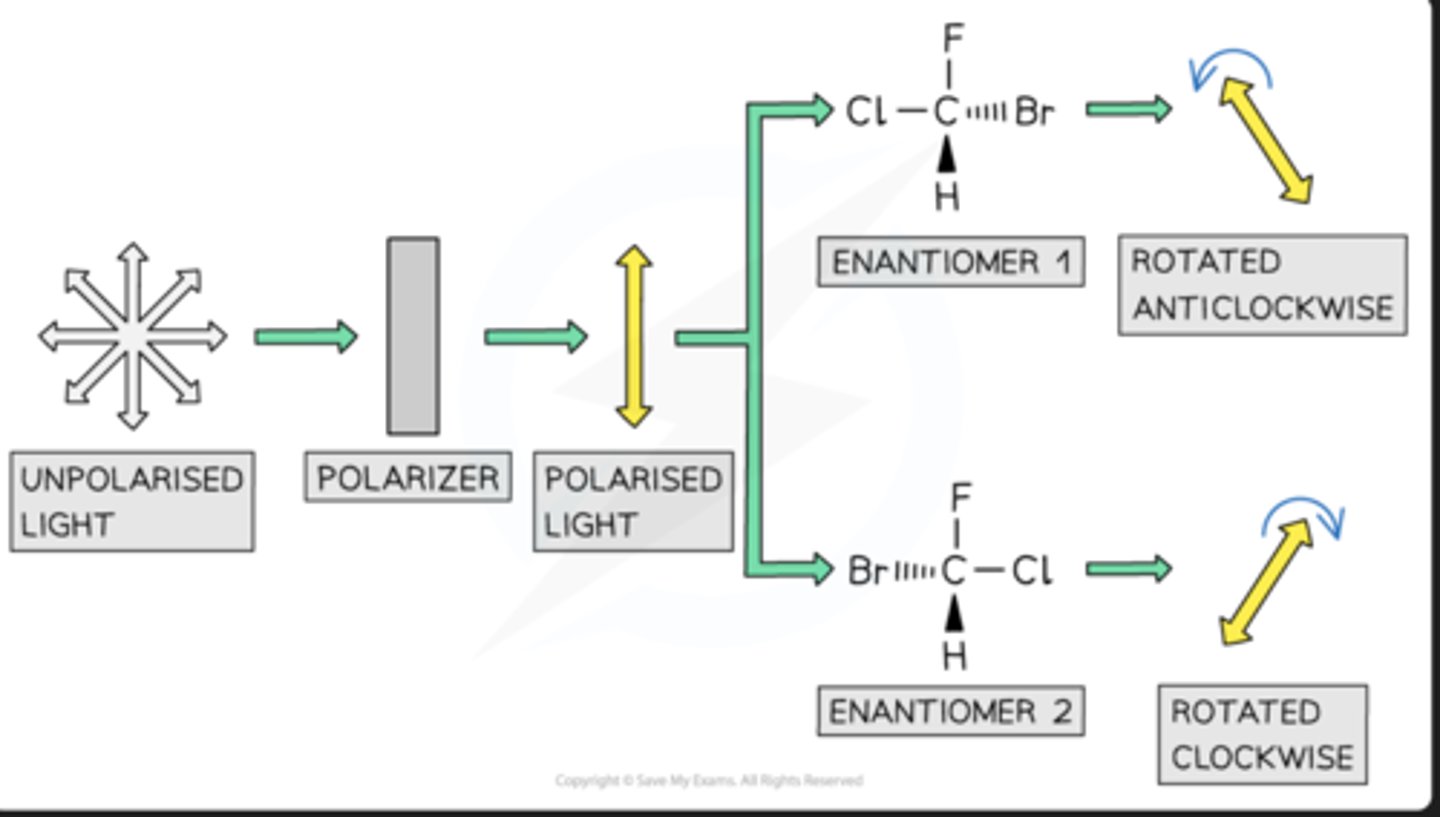
Compounds with one chiral centre (chiral molecules) exist as two optical isomers, also known as enantiomers
Just like the left hand cannot be superimposed on the right hand, enantiomers are non-superimposable
Enantiomers are mirror images of each other
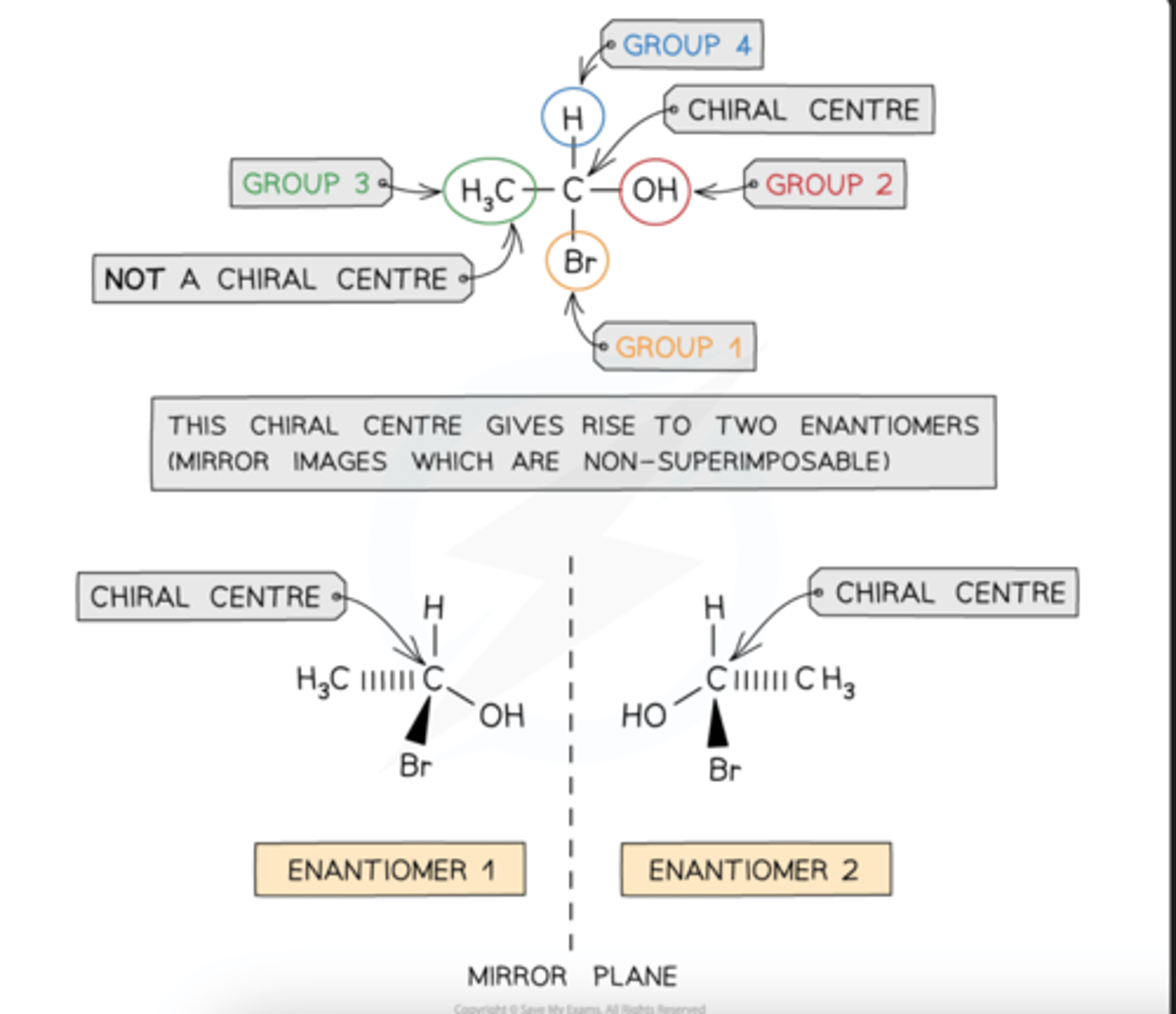
Diastereomers
are compounds that contain more than one chiral centre
Diastereomers are not mirror images of each other because each chiral carbon has two isomers
Optical isomers have identical physical properties, with one exception
Isomers differ in their ability to rotate the plane of polarised light (waves vibrate in one plane only)
The major difference between the two enantiomers is:One enantiomer rotates plane polarised light in a clockwise manner and the other in an anticlockwise fashion
R=clockwise, L=counterclockwise
The rotation of plane polarised light can be used to determine the identity of an optical isomer of a single substanceFor example, pass plane polarised light through a sample containing one of the two optical isomers of a single substanceDepending on which isomer the sample contains, the plane of polarised light will be rotated either clockwise or anti-clockwise by a fixed number of degrees