meow chem/ochem/physics
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
amino acids pka values: carboxyl n amino group
carboxyl: 2.5
amino: 9.5
what happens to the buffering region of the titration curve of ionizable amino acids?
NORMAL: 2 pka (buffering regions)
ionizable amino acids: 3 pka
HER DYCK
what does pka refer to
half of desired group are protonated
2 things that can disrupt tertiary structure of protein
temperature
urea concentration (interferes w hydrogen bonding, exposes hydrophobic groups, causes denaturation)
hydrostatic pressure
HIGH to LOW pressure
interstitial fluid to blood
osmotic pressure
LOW to HIGH pressure
blood to interstitial fluid
temperature, quantity of molecules, and kinetic energy KE of an ideal gas
TEMP n KE = directly proportional
TEMP n QUANTITY of molecules = inversely proportional
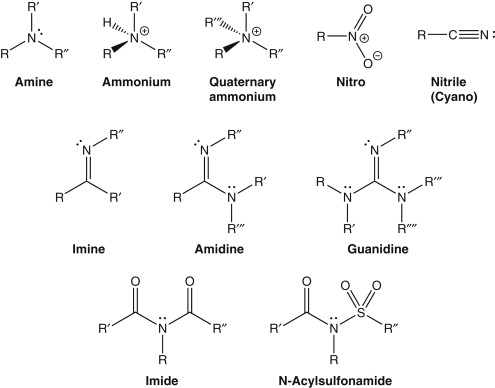
amide vs. imine vs. imide (all involve Nitrogen)
Amide: derived from COOH
Imine: formed from aldehydes or ketones
Imide: derivative of dicarboxylic acids
large area + delocalization of electrons via conjugated double bonds leads to what?
MORE stability when MORE Pi bonds, large system, and greater delocalization
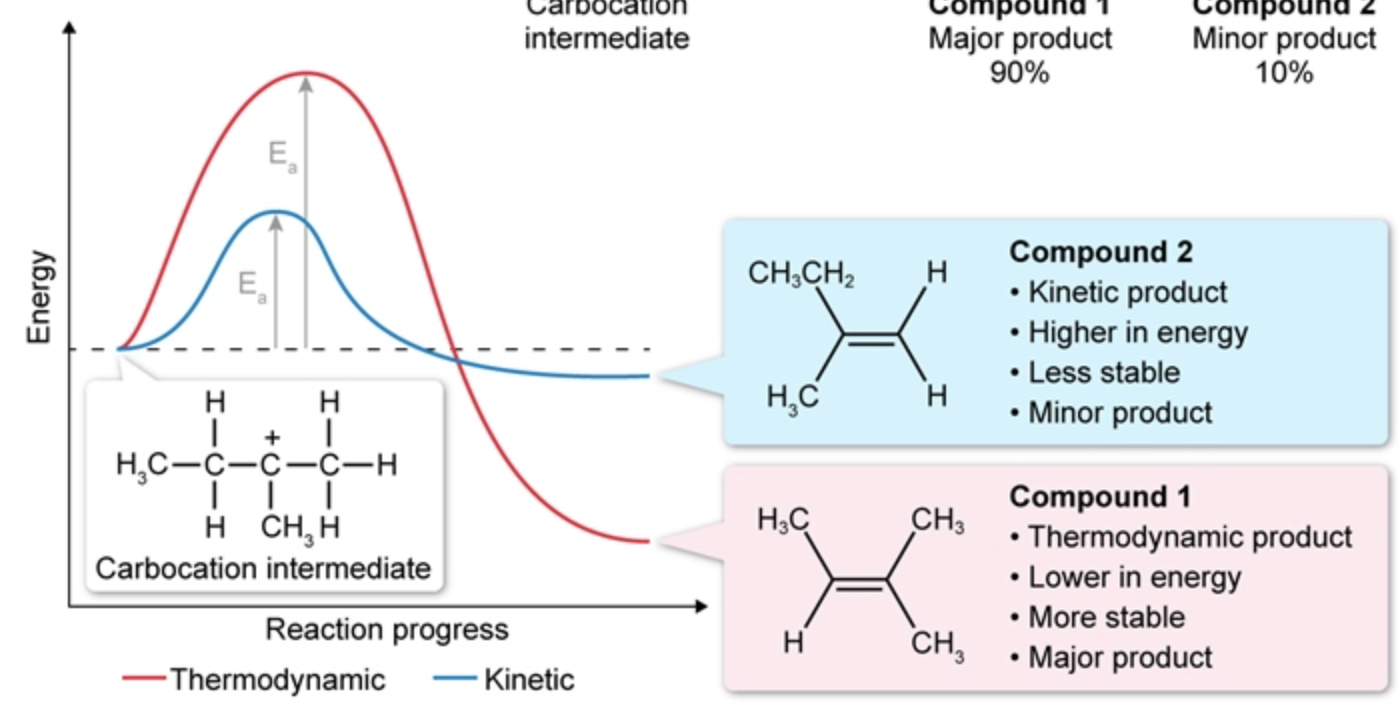
thermodynamic vs kinetic product properties (stability, activation energy Ea, energy level)
compared to kinetic, thermodynamic is:
MORE stable
HIGHER Ea
LOWER energy
current n charge equation
I = Q/T or current = charge/time