Feeding and NG
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
What is adult hippocampal neurogenesis (AHN) and what is it important for? (3)
Ongoing process of generating functionally integrated neurons from progenitor cells in the adult brain.
Affects brain structure/plasticity – contributes to normal brain approx. 7 weeks.
AHN is essential for pattern separation memory — distinguishing between similar contexts.
Eg someone with PTSD
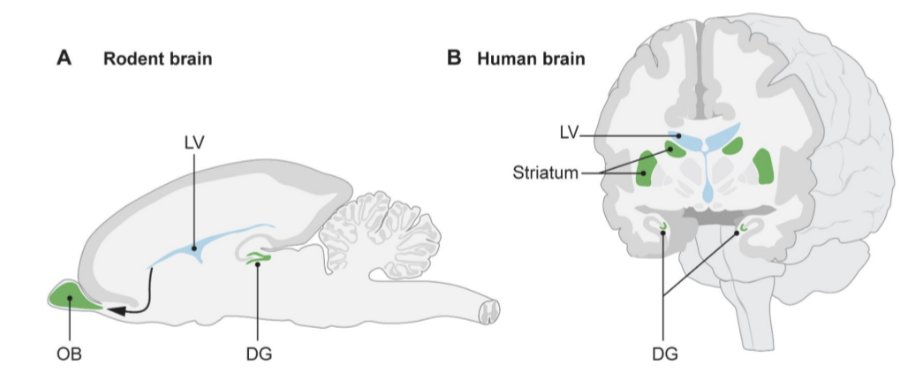
Where does ANH occur?
Happens in the subventricular zone (SVZ) of lateral ventricles and dentate gyrus (DG) of the hippocampus.
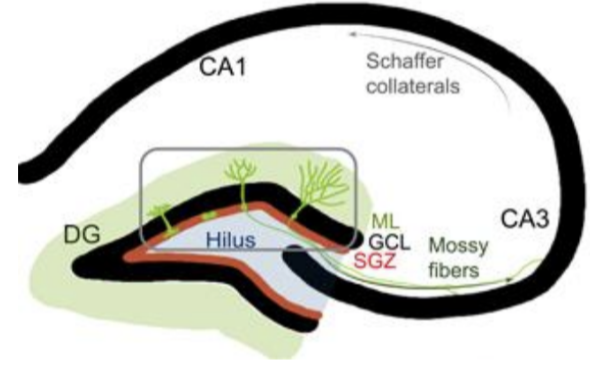
DG contains the subgranular zone (SGZ) — a neurogenic niche with self-renewing, multipotent stem cells.
What’s the red line showing?
Subgranular zone (SGZ) (neurogenic niche wiht self-renewing, multipotent stem cells
What factors affect adult hippocampal neurogenesis (AHN)?
Decrease AHN:
Age
Stress
Depression
Dementia
Increase AHN:
Exercise
Enriched environments
Antidepressants
Calorie restriction (CR)
What key evidence from 2 studies supports the occurrence of adult neurogenesis in humans? (7)
Fred Gage (1998):
Used BrdU labeling in postmortem hippocampal tissue from cancer patients.
Found BrdU-positive cells in the dentate gyrus (DG), indicating new neuron formation.
Proved adult hippocampal neurogenesis in humans.
Jonas Frisén (2013):
Used carbon-14 dating from nuclear bomb testing to track new neuron birth.
Carbon-14 incorporated into DNA of dividing cells.
Showed over 700 new neurons/day in adult DG → ~1.75% turnover/year.
Confirmed lifelong neurogenesis in the human hippocampus.
How does calorie restriction (CR) enhance neurogenesis and memory, and what role does ghrelin play? (6)
Response to CR is conserved across species and linked to reduced disease incidence, increased longevity, associated with improvements in learning + memory
Activates AMPK pathways → boosts mitochondrial biogenesis and autophagy → improved cell metabolism and insulin sensitivity.
CR is thought to mediate its beneficial effects by promoting hippocampal plasticity and neurogenesis, and one possible mediator is thought to be ghrelin.
CR elevates circulating ghrelin (hunger hormone) — other GI hormones are reduced.
Ghrelin may mediate the beneficial effects of CR on mnemonic function (increase memory retention)
Known as perinatal programming when early-life feeding affects long-term brain outcomes.
What is ghrelin?
Ghrelin is a 28 amino acid orexigenic peptide produced by PD/1 cells in the stomach.
Known as the “hunger hormone”, it regulates food intake and calorie homeostasis.
It stimulates GH (growth hormone) release from the pituitary.
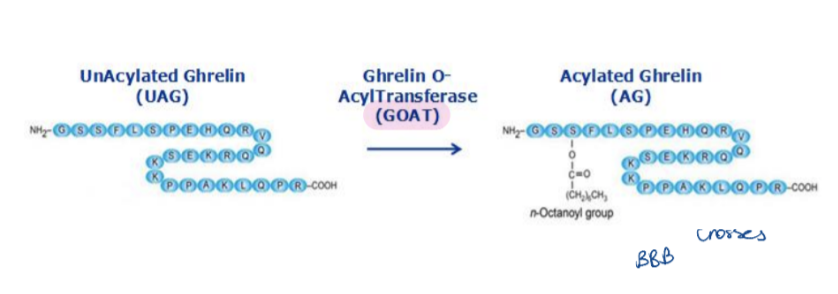
Exists in two forms:
Acyl ghrelin (AG) – active form
Unacylated ghrelin (UAG) – inactive form
AG is octanoylated on the 3rd serine residue by the GOAT enzyme (ghrelin O-acyltransferase) in the stomach.
AG crosses the blood-brain barrier (BBB) and acts via the GHSR1a receptor (a G-protein-coupled receptor)
How does ghrelin fluctuate throughout the day around meals?
Ghrelin levels rise before meals (breakfast, lunch, dinner), triggering hunger.
Levels drop sharply after eating.
This cycle repeats, showing a pre-prandial rise and postprandial fall.
Ghrelin acts as a short-term regulator of hunger, peaking in anticipation of meals and falling once energy intake begins
Where and on what cell types is the ghrelin receptor (GHSR) expressed, and which is it not expressed on?
Cells/areas expressed on (3)
Cells not expressed on (3)
GHSR (GHSR1a) is highly expressed in the granule cell layer of the dentate gyrus (DG) in the hippocampus.
It is not expressed on:
Type 1 radial glia-like neural stem cells (Nestin⁺)
Type 2 neural stem/progenitor cells (Sox2⁺)
Actively proliferating cells (Ki67⁺)
Widely expressed on mature granule neurons (NeuN⁺) in the DG.
Also expressed in other brain regions like the basolateral amygdala and lateral entorhinal cortex.
Does ghrelin promote hippocampal stem cell proliferation and adult hippocampal neurogenesis (AHN)? (6)
No, ghrelin does not promote hippocampal stem cell proliferation, but it does enhance adult hippocampal neurogenesis (AHN) via mature neurons.
Ghrelin promotes AHN through indirect, neurogenic effects:
↑ BrdU⁺/NeuN⁺ newborn neurons in the rostral DG
↑ Pattern separation memory (SLR task)
Study details:
Rats treated with 10 µg/kg acyl-ghrelin (i.p., 2 weeks)
BrdU (days 5–8) marked dividing cells
Cognitive testing on day 20–28 showed improved memory
No stem cell pool depletion observed
🚫 No significant effect on:
Self-renewing stem cells (BrdU⁺/Sox2⁺)
New astrocyte formation (BrdU⁺/Sox2⁺/S100β⁺)
✅ Conclusion:
Ghrelin enhances AHN and memory by acting on mature neurons—not by increasing stem cell proliferation or exhausting progenitor pools.
Does calorie restriction (CR) have a similar neurogenic effect as acyl-ghrelin (AG) on the brain? Explain
Yes or no summary
Key markers (2)
Quantitative data (2)
Conclusion (2)
Yes, calorie restriction mimics the neurogenic effects of acyl-ghrelin (AG) by promoting activity in dentate gyrus neurons.
🧠 Key markers:
c-Fos (neuronal activity marker): ↑ by AG but not by CR
Egr-1 (immediate early gene linked to neurogenesis): ↑ by both AG and CR
📊 Quantitative data:
c-Fos⁺/GHSR⁺ cell numbers:
AG and AG+CR significantly ↑ compared to vehicle
CR alone had no significant effect on c-Fos
Egr-1⁺/GHSR⁺ cell numbers (rostral DG):
AG, CR, and AG+CR all significantly ↑ compared to vehicle
AG+CR had the strongest effect
✅ Conclusion:
Calorie restriction promotes neurogenesis via Egr-1 upregulation in GHSR⁺ neurons, similar to AG.
However, AG uniquely increases c-Fos, suggesting it may have a broader or more immediate impact on neuronal activity.
Does calorie restriction (CR) enhance adult hippocampal neurogenesis (AHN) via the ghrelin receptor (GHSR)?
Yes/no summary
Method (2)
Findings (2)
Conclusion
Yes — calorie restriction enhances AHN in a GHSR-dependent manner.
Method:
Mice were calorie restricted to 70% of ad libitum intake for 14 days.
BrdU (days 4–7) labeled dividing cells.
Behavioural tasks assessed memory on days 31–45.Findings:
In WT mice, CR increased BrdU⁺/NeuN⁺ cells in the dentate gyrus by 52%, indicating more newborn neurons.
In GHSR⁻/⁻ knockout mice, this effect was abolished — showing CR’s neurogenic effects depend on GHSR signalling.
Conclusion:
CR enhances AHN, but this enhancement requires ghrelin receptor (GHSR) activity.
What effect does unacylated ghrelin (UAG) (inactive form of ghrelin - 80-85% circulating ghrelin) have on AHN?
Summary 1 line
Key effects (4)
Conclusion
UAG impairs adult hippocampal neurogenesis (AHN) in both WT and GOAT⁻/⁻ mice.
↓ Proliferation:
– 40% reduction in Ki67⁺ proliferating cells in WT and GOAT⁻/⁻ mice (↓ cell division in the dentate gyrus).↓ Immature neurons:
– Significant reduction in DCX⁺ cells (immature neurons), suggesting impaired neurogenesis.↓ Newborn neurons:
– Fewer BrdU⁺/DCX⁺ cells in WT and GOAT⁻/⁻ (less neuronal differentiation and survival).Cognitive effects:
– GOAT⁻/⁻ mice show spatial memory deficits (Y-maze, SLR tasks).
– These deficits were rescued by 7-day acyl-ghrelin treatment, and effects lasted 28 days post-treatment.
Conclusion:
UAG has a negative impact on hippocampal plasticity and neurogenesis. Its effect is independent of GOAT and is linked to reduced proliferation, immature neurons, and impaired spatial memory — all reversible with AG treatment.
Can ghrelin be used aas a biomarker for cognitive decline? Explain (3)
Yes, ghrelin—particularly acyl-ghrelin (AG)—shows promise as a biomarker for cognitive decline.
In individuals with Parkinson's Disease Dementia (PDD):
Altered ghrelin dynamics:
PDD patients had a significantly reduced post-prandial total ghrelin and AG response after a 300 kcal meal compared to age-matched controls.
Fasting and post-prandial AG/TG ratios:
PDD patients showed significantly lower ratios of AG (as a % of total ghrelin) in both fasting and 180 min post-prandial states.
Fasting AG/TG ratio significantly reduced (p = 0.0302)
Post-prandial AG/TG ratio also reduced (p = 0.0144)
Conclusion:
PDD is associated with disrupted ghrelin regulation, especially AG.
Thus, reduced AG levels and ratios could potentially serve as biomarkers of cognitive decline, specifically in Parkinson’s disease dementia.
Study details: 4 groups: Controls, PD, PD + mild cognitive impairment (MCI), and PDD. Blood collected at baseline and at 5–180 min post-meal to assess ghrelin dynamics
How do different feeding patterns affect AHN and behaviour, and is this effect GHSR-dependent?
Meal feeding (3x a day) effects (2)
What does loss of GHSR singalling lead to? (1)
In WT mice
In LoxTB-GHSR mice (GHSR signalling blocked)
Meal feeding (3x/day) promotes neurogenesis and anxiolysis in WT mice, but not in LoxTB-GHSR mice.
Meal feeding promotes new neuronal cell survival in the
presence of GHSR signalling
Loss of GHSR signalling reduces discrimination memory
In WT mice:
Meal-fed mice had ↑ BrdU⁺/NeuN⁺ neurons (i.e., new neurons).
Grazing and meal-fed groups also had ↑ BrdU⁺/NeuN⁻ cells (i.e., other new cells).
Meal feeding improved memory in the Object-in-Place (OIP) task.
Meal feeding increased time in the inner zone, entries, and movement in Open Field (OF) test → anxiolytic effect.
In LoxTB-GHSR mice (GHSR signalling blocked):
No difference in new neurons or other new cells between feeding patterns.
No improvement in discrimination memory.
Reversal of anxiolytic effects: meal-fed mice spent less time in the inner zone and moved more → anxiety-like behaviour.
Conclusion:
Meal feeding enhances neurogenesis, memory, and reduces anxiety—but these effects require intact GHSR signalling.
Summary and implications - What are the key implications of ghrelin and calorie restriction on AHN and cognitive function? (6)
Calorie restriction (CR) and ghrelin both enhance adult hippocampal neurogenesis (AHN) and improve memory.
These effects are GHSR-dependent, with GHSR highly expressed in mature neurons in the dentate gyrus (DG), as well as in the basolateral amygdala and lateral entorhinal cortex.
CR is neuroprotective—shown to reduce cognitive decline in Alzheimer's models (Halagappa et al. 2007).
→ Endogenous ghrelin may mediate this effect (hypothesis).
Unacylated ghrelin (UAG) impairs both AHN and memory.
Emerging evidence links Parkinson’s Disease (PD) and dementia with altered ghrelin signalling.
→ Raises possibility that ghrelin/CR could be therapeutic for cognitive impairment (Bayliss 2013).
Feeding patterns (e.g. meal feeding) crucially shape ghrelin’s neurogenic impact.
Bottom line:
🧠 Ghrelin connects metabolism and cognition, with increasing interest in its role as a blood-borne regulator of brain plasticity.