BCMB2001: biochemistry
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
111 Terms
what is NAD+?
nicotinamide adenine dinucleotide → accepts H+ to become NADH (oxidises)
what is FAD?
flavin adenine dinucleotide → accepts 2H+ and 2e- to become FADH2
what is CoA?
carrier of acyl groups
traps metabolites within the cell (e.g. FAs)
what is the formula for energy charge? why is AMP the key impacting factor?
AMP will make the key change to this (as ATP, ADP on both sides of the fraction)
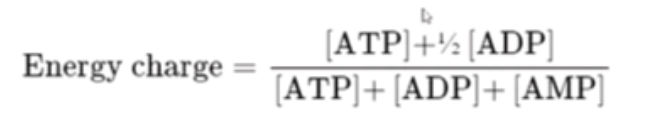
what is the difference between kinases, phosphatases and phosphorylases?
kinase - catalyse a phosphorylation reaction (adds a phosphate group to a substrate)
phosphatases - catalyse dephosphorylation reactions (removes a phosphate group from a substrate)
phosphorylases - catalyse a phosphorolysis reaction (uses a phosphate group to break a substrate apart)
what is the difference between synthases, synthetases and dehydrogenases?
synthases - catalyse condensation reactions in which no nucleotide triphosphate is required (no ATP)
synthetases - catalyse condensation reactions that require a nucleotide triphosphate
dehydrogenases - catalyse oxidation-reduction reactions, usually involve NAD+/FAD as cofactors
how does ATP synthase operate?
H+ flows through a channel in the inner mitochondrial membrane
causes another protein to rotate → interacts with the subunits of the ATP synthase to generate ATP from ADP and phosphate
explain the 4 key steps of oxidative phosphorylation
fuels (fats, carbs), are oxidised by stripping H+ and e-
carriers strip these and take them to the ETC
movement of these down the ETC provides energy to pump protons out of the mitochondria
only way the protons can come back in is through ATP synthase → generates ATP
what are the 7 big concepts with fuel oxidation?
the H/e- carriers are in short supply - if there were loads we could just keep burning fuels, wouldn't need to do any work
ADP is in short supply - need to do work to make ADP
ATP is really stable - UNLESS it is used to drive a chemical reaction, it won't break down
the inner mitochondrial membrane is impermeable to protons - the only way protons can get back into the matrix is through ATP synthase
protons only flow into the matrix if the ATP is being made
the proton pumps dont work if the proton gradient is very high
no proton pumping, no H/e- movement down the ET chain
what are the key features of fatty acids?
fatty acids - fully reduced carbons, stored as triglycerides, hydrophobic
energy dense, huge stores
CAN’T be used by the brain
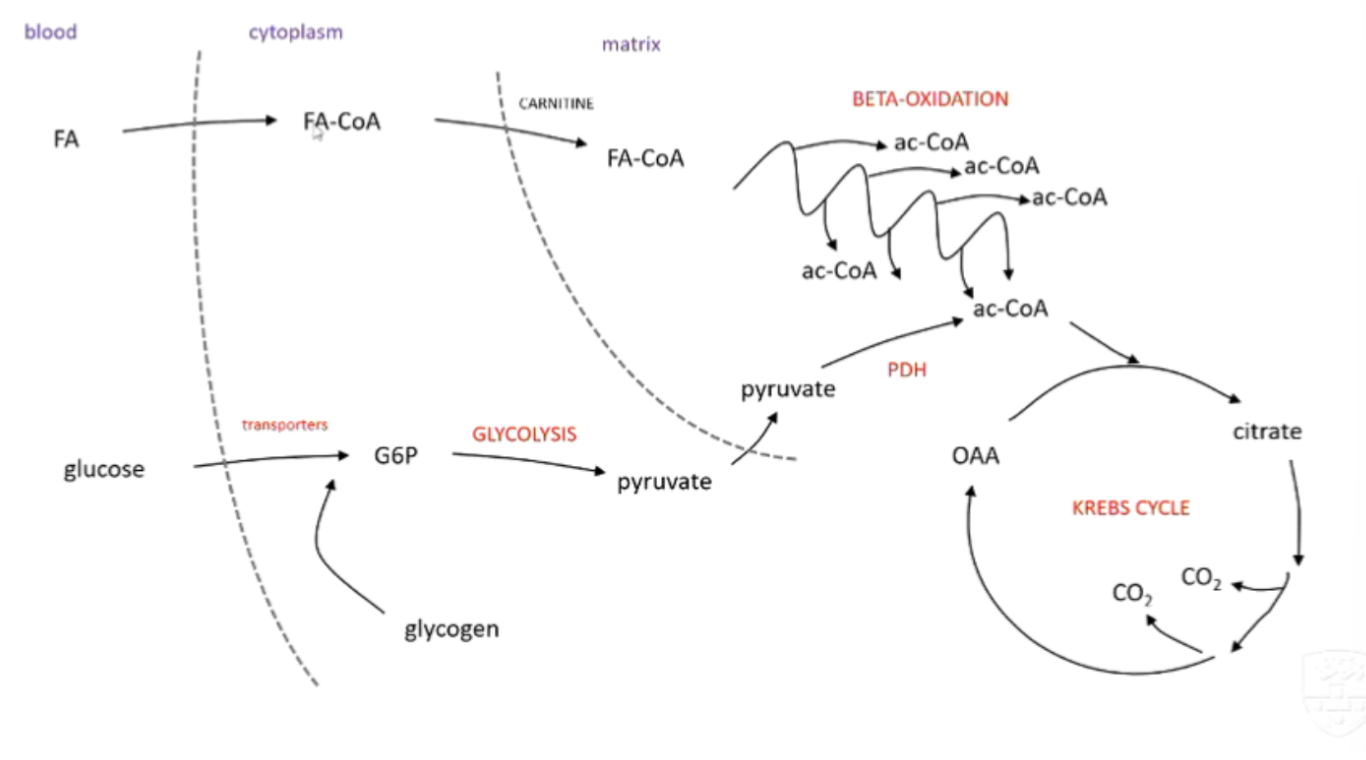
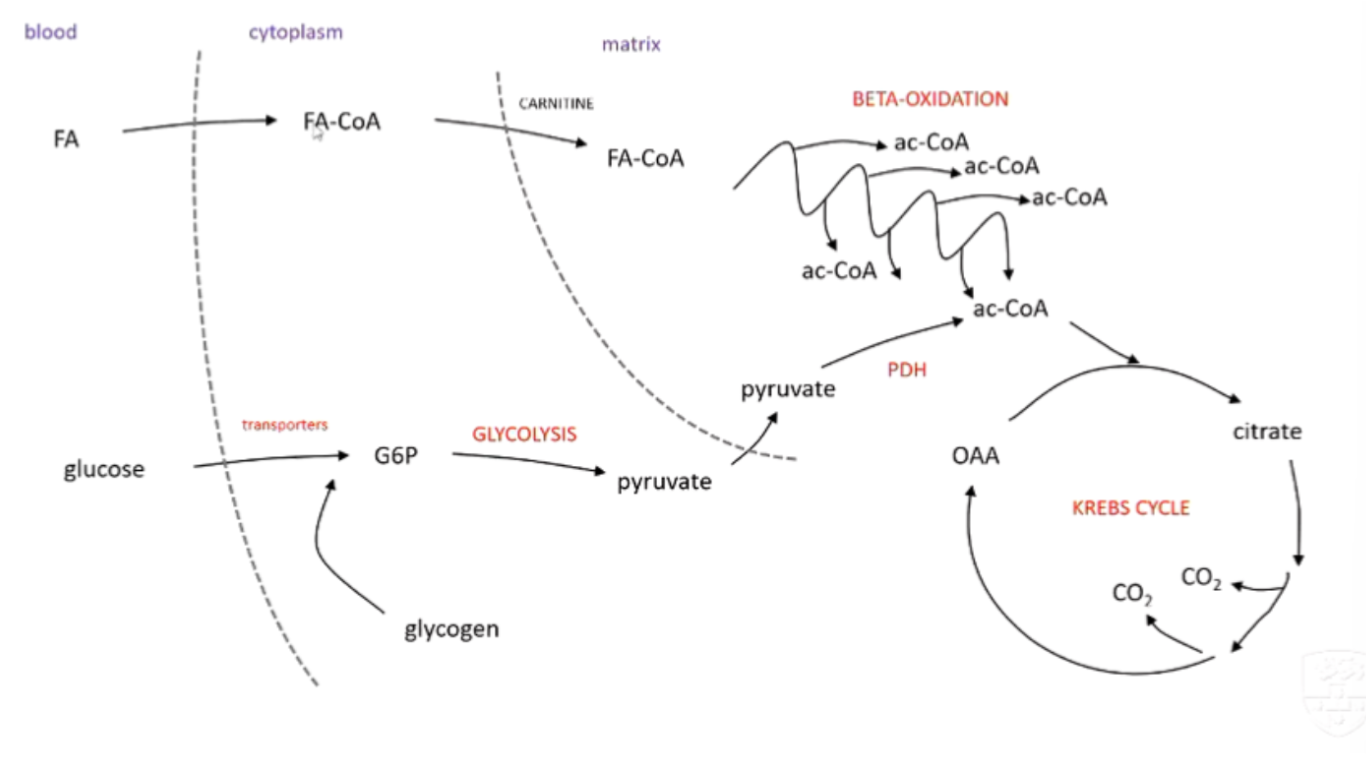
explain the process of beta-oxidation
breaking down fatty acids → acetyl CoA
carnitine carrier transports fatty acid from cytoplasm → mitachondria
H/e- ripped out by FAD and NAD+
fatty acid part loses an acetate chunk → put into Krebs cycle
cycle repeats to fully oxidise fatty acids
what are the key features of glucose?
reasonably reduced, hydrophilic, stored as glycogen (low stores ~300g)
inefficient: 16kJ/g but only 6kJ/g wet
can be used by all tissues → brain has priority (can’t use fats)
what are the key features of glycolysis?
occurs entirely in the cytosol, doesn’t need oxygen
very fast BUT very inefficient
products:
small amount of ATP → irrelevant compared to oxidative phosphorylation
pyruvate → must be transported into mitochondria for full oxidation
what are the key features of proteins?
body has 5-10kg, but not ‘stored’ for energy
energy release: 17kJ/g
making proteins uses lots of energy
amino acid catabolism → pyruvate, Acetyl CoA or Krebs Cycle
is protein a suitable fuel source for the body?
only when necessary: need proteins as they have a specific function
also inefficient: need to use ATP to remove amine groups
think of burning a fancy well crafted chair for heat
is oxygen used in any of these processes?
no
explain how fuels combined with oxygen to produce water and CO2?
fuels oxidized: stripped of H/e⁻
oxygen: final electron acceptor in ETC
CO₂: released in Krebs Cycle
H₂O: formed as electrons combine with O₂ and protons at end of ETC
how will fuel oxidation processes be impacted by a reduction in ATP consumption?
less ATP consumption → ATP synthase slows, builds up proton gradient → ETC slows → H/e⁻ carriers remain reduced
result: fuel oxidation processes SLOWED
how will fuel oxidation processes be impacted by an increase in ATP consumption?
more ATP consumption → ATP synthase active, protons flow → ETC active → H/e⁻ carriers regenerated
result: INCREASED catabolic activity to match energy demand
how does muscle contraction use ATP?
muscle contraction uses ATP for actin and myosin interaction: filaments sliding across each other
the faster the contraction, the faster the use of ATP
how do muscles use ATP at rest?
even at rest, muscle is using ATP: maintaining ion gradients, sarcoplasmic reticulum and Ca2+ concentration
compare type 1 ans 2b muscle fibres
type 1 (slow-twitch, red) | type 2b (fast-twitch, white) |
contracts slowly | contracts fast |
many mitochondria | few mitochondria |
good blood supply | poor blood supply |
full of contractile filaments |
what happens to oxidative processes as we exercise?
as we exercise: everything speeds up so we can replenish our ATP stores
increased ATP consumption, so more ADP (ATP → ADP)
ATP synthase uses ADP to make more ATP → more protons enter mitochondrial matrix
more H/e- can flow down because of the lower gradient
NADH and FADH2 can drop their H/e- quicker
compare ATP stores to turnover
ATP stores are small (1g/kg) so turnover is rapid (1kg/kg) - very high compared to at rest
how does muscle glucose use affect BGL, insulin and glucagon?
glucose used → lowers BGL → lowers insulin, increases glucagon.
how does the body process this glucose (glycolysis)?
transporters move to cell surface to allow glucose to enter → glucose phosphorylated to G6P →
glycolysis → 2 pyruvate molecules → pyruvate dehydrogenase convert pyruvate to acetyl-COA that will feed the krebs cycle
predict the effect of the low insulin and high glucagon on target tissues and blood fuel levels
liver: glycogen breakdown → glucose release
adipose tissue: fat breakdown → fatty acids released → BGL restored
why does glucose need to be recycled?
glucose/glycogen stores are limited → need to be recycled
fatty acids cannot be converted into glucose; brain needs glucose
how is glucose recycled? how does PDH stimulate glucose recycling?
glucose recycling:
fatty acids join to CoA: used as primary supply of CoA → inhibits PDH from converting pyruvate → CoA, so:
glucose → pyruvate → lactate → back to liver → glucose (gluconeogenesis)
compare initial fuel use in exercise to a few mins later
initially: glucose used
after several minutes fatty acids take over → released from white adipose tissue
glucose still gets into the muscles but is only taken as far as lactate → goes to the liver for re-synthesis of glucose (gluconeogenesis)
how is fuel oxidation increased in gentle exercise?
need to elevate the rate of ATP generation through greater availability of ADP
increase ATP synthase
dissipate the proton gradient (our charged battery is being used)
increase electron transport (because now it can)
increase availability of H/e- strippers
→ fuel oxidation can increase
how does faster pace affect acetyl CoA demand? how does this affect PDH?
faster pace: increased acetyl-CoA demand
fat oxidation can’t hand alone: PDH inhibition lifted and glucose oxidation used
compare fuel use in gentle vs moderate exercise
gentle: mostly fatty acids, some glucose → lactate
moderate: maxed-out fat oxidation + increased glucose oxidation (PDH inhibition lifted) → more liver glycogen used; less glucose recycled
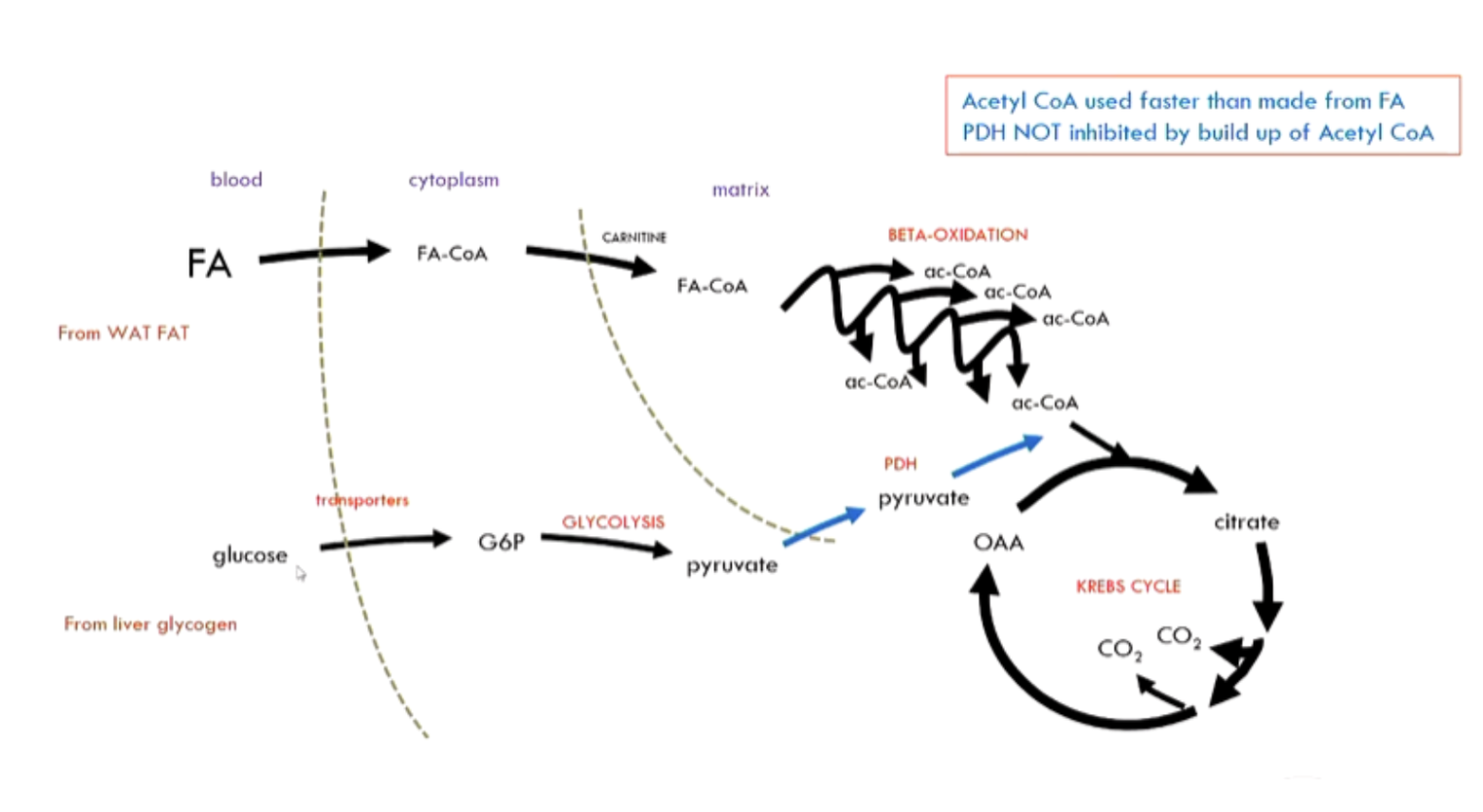
how does fuel use change from gentle to strenuous exercise?
fatty acids maxed out, limits on used of glucose → so glycogen is broken down (glycogenolysis)
what are the advantages and disadvantages of glycogen being used in very strenuous exercise?
glycogen used in very strenuous exercise: oxidative phosphorylation can’t produce enough ATP
advantages: glycolysis very fast
disadvantages: very inefficient (2 ATP per glucose)
how are fuels used in strenuous exercise?
fat oxidation at max, BGL delivery & oxidative phosphorylation too slow
muscle glycogen → glycolysis → lactate
how are fatty acids transported in blood and then into cells?
fatty acids are hydrophobic, can transport in blood if bound to albumin (protein)
entry into cells mostly by passive diffusion, then associate with fatty acid-binding proteins, and are trapped by attaching CoA
uses a lot of energy
how is carnitine used to aid fatty acid transport?
cytosolic FA-CoA → carnitine acyl-transferase I swaps CoA for carnitine
carnitine-FA enters mitochondria (Fa-CoA can’t)
inside: carnitine acyl-transferase II swaps back to CoA
carnitine goes back out and is recycled
what does FAD oxidise in the first step of FA-CoA oxidation?
first H/e- stripping: FAD oxidises –CH2–CH2– to –CH=CH–
FAD becomes FADH2
occurs between alpha and beta carbon - can then be hydrated to form an -OH group
what does NAD+ oxidise in the second step of FA-CoA oxidation?
second H/e- stripping: NAD⁺ oxidises –CH–OH to –C=O
NAD⁺ becomes NADH
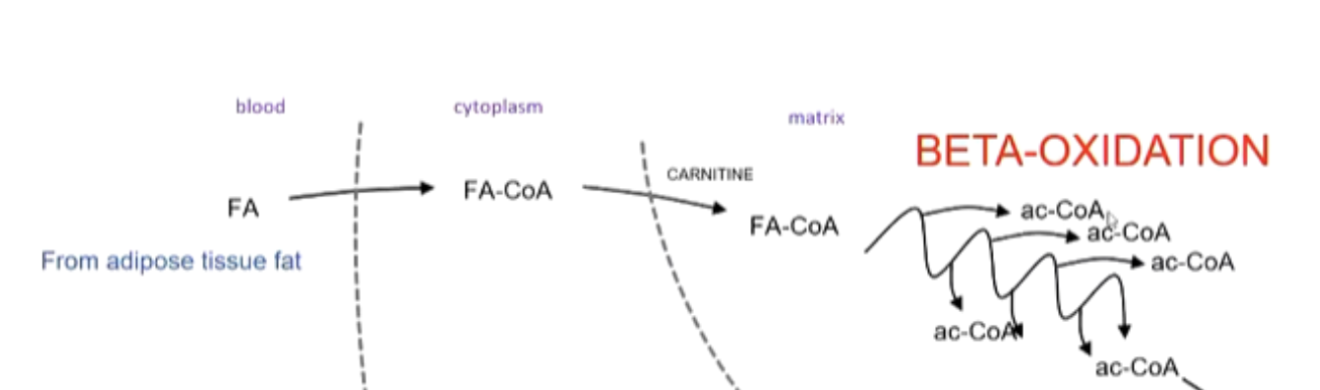
outline the process of Beta-Oxidation
hormone signalling causes fatty acids to go from adipose tissue → bloodstream → cell
trapped in the cytoplasm by bonding to CoA (FA-CoA)
CoA taken off → Carnitine takes it into mitochondria → CoA swapped again with Carnitine
FA-CoA goes through multiple rounds of Beta-Oxidation
products: 1 Ac-CoA, FADH2 and NADH per round (+ 1 Ac-CoA left at the end)
what are the two main ways pyruvate can be used?
aerobic: pyruvate → acetyl-CoA via PDH, enters Krebs cycle
anaerobic: pyruvate → lactate (regenerates NAD⁺ for glycolysis), lactate can be recycled to glucose in liver
what are the key features of glycolysis?
all tissues can perform glycolysis
wholly cytosolic, anaerobic
fast BUT inefficient (2ATP)
red blood cells have to rely on this (don't have a mitochondria)
outline the strategy of the Krebs cycle
fully oxidise acetyl-CoA to CO2 → Produce lots of NADH, FADH2, even an ATP (not directly - GTP)
regenerate oxaloacetate to keep cycle turning
describe the process of the Krebs Cycle
Acetyl-CoA (2C) + oxaloacetate (4C) → citrate (6C), which undergoes a series of reactions
During the cycle: 2C come in, 2C released
Products: 3 NADH, 1 FADH2, 1 GTP (about 10 ATP per acetyl-CoA), 2 CO
What is the purpose of the Krebs Cycle?
NOT to make ATP (only get 1 in the form of GTP)
generates lots of NADH and FADH2
What are the regulatory features of glycolysis, beta-oxidation and the Krebs cycle?
Regulated by availability of cofactors (NAD+, FAD, ADP) → more of these = goes faster
Inhibited by a high ‘energy charge’ (ATP/ADP ratio)
How can oxygen consumption or carbon dioxide production give an estimate of energy expenditure?
coupling principle: Fuel oxidation rate = ATP use (demand-driven)
hence rate of O2 consumption and/or CO2 production estimates energy expenditure
What is the result of uncoupling? Why is this bad?
H+ no longer needs to pass through ATP synthase → The proton gradient would dissipate → no ATP produced
No back-pressure to stop H+ pumping
No restriction on H/e- movement down the ETC to oxygen
Carriers are immediately reoxidised, can continue oxidising fuels → Consume fuels and O2 super fast whilst generating no ATP (product lots of heat - sweating)
What is DNP?
DNP = Dinitrophenol. Weak acid that diffuses across mitochondrial membrane when protonated (becomes hydrophobic)
Cytoplasm: lots of H+ → join onto DNP, which diffuses into the mitochondria.
Since there is not much H+ in mitochondria, H+ will detach, process repeats
collapses proton gradient.
Increases O₂ consumption, NAD⁺ regeneration, but no ATP made.
What is UCP-1?
UCP-1 = Uncoupling protein 1 (Thermogenin). Natural uncoupler.
Found only in brown adipose tissue
Activated by noradrenaline, stimulates fatty acid release and opens H+ channel.
Short circuit ATP synthase by opening inner pore of UCP-1 - generates heat, no ATP
How is brown adipose tissue important for mammals, including humans?
Generates heat - Especially in small mammals and hibernating animals
In humans: high presence in neonates. Involved in adaptive thermogenesis (e.g., cold exposure)
What is the flow of electrons through the ETC?
NADH drops electrons at complex I
FADH2 drops electrons at complex II
Complex I and II → Q → Complex III →Complex IV
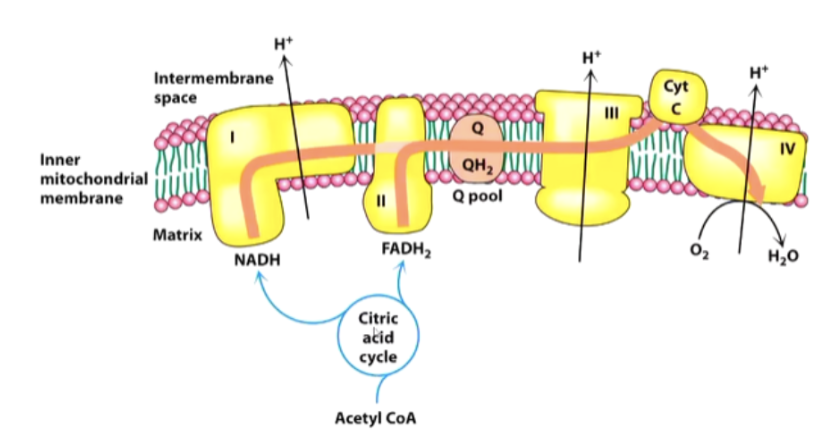
How do proteins affect the ETC structure and transport of H/e-?
Each complex consists of many proteins, which maintain their shape, and have a prosthetic group to transport H/e-
Proteins have H+ expelling reactions on outside, H+ consuming reactions on the matrix side
What are the properties of UQ?
Electrons move around in Complex I from one prosthetic group to another until they reach the Q pool (where UQ is)
UQ: Hydrophobic, lives in membrane
Where does UQ receive H/e- from?
Accepts 2H + 2e⁻ → UQH₂. Receives from Complex I, II, β-oxidation, G3P shuttle.
How many protons are pumped per NADH and FADH 2? Where are they pumped from?
Per NADH: 10 H⁺ pumped (4 from Complex I, 4 from III, 2 from IV).
Per FADH₂: 6 H⁺ pumped (4 from III, 2 from IV) → less ATP from this
How does the glycerol 3-phosphate shuttle work?
Glycerol 3-phosphate shuttle: Transfers e- from cytosolic NADH to FAD in mitochondria
Bypasses Complex I, enters ETC at Complex III → only 6 H⁺ pumped per NADH
How does the malate aspartate shuttle work?
Malate aspartate shuttle: Cytosolic NADH reduces oxaloacetate → malate, which enters matrix
Malate is reoxidised → NADH in matrix → enters Complex I (10 H⁺ pumped as normal)
What are the four separate routes that feed into UQ?
Complex I (NADH via malate shuttle)
Complex II (FADH₂ from succinate)
Glycerol 3-phosphate shuttle
First step of beta-oxidation
How are free radicals formed and how can they be prevented from forming?
Electrons in the UQ pool can react with oxygen → produces free radicals (very dangerous, cause DNA mutation)
Less likely to form if complex III is vacant
How much ATP is produced per NADH?
ATP synthase: Using the H+ gradient to make ATP
Movement of 3 protons = generation of 1 ATP → 10 H+ per NADH, therefore 3.3 ATP from 1 NADH
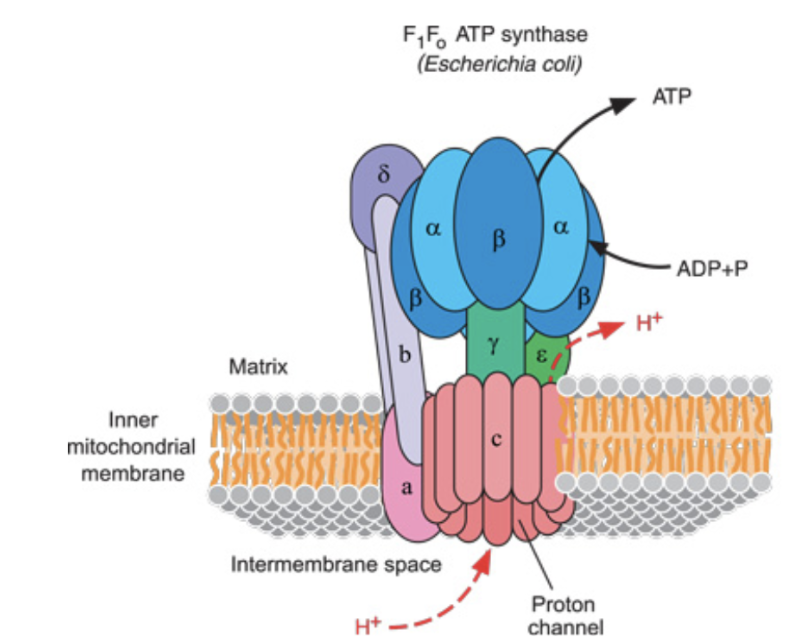
Explain the mechanism by which the ATP synthase produces ATP.
This causes Beta subunit of F1 to:
Accept ADP and Pi
React them to give ATP
Release the ATP
Every time 3 H+ come in, the beta-subunit (there are 3) changes conformation, and forms 1 ATP
How does Rotenone impact the ETC?
Rotenone: Inhibits at complex I
H+ pumping stops, no ATP
Everything downstream is oxidised - won’t reduce because no electrons are coming down for it to capture
How does Cyanide, azide and CO impact the ETC?
Cyanide, azide, carbon monoxide: Inhibits at complex IV
H+ pumping stops
BUT Everything upstream stays reduced
How can inhibitors of the ETC be fixed?
Methylene blue: Acceptor from complex IV before blockage point → allows ETC to continue
What are the 4 main fuel stores in the body?
Fuel stores:
Adipose tissue - 15kg
Proteins (mainly muscle) - 6g
Glycogen (muscle, liver) - 0.2g
Circulating fuels (Glucose, fatty acids, etc) - 0.2g
What are the 3 key parts of the glucose homeostasis strategy?
Glucose homeostasis strategy:
Conservation – don’t use glucose unless you must
Recycling – via Cori Cycle (lactate → glucose instead of fully oxidising glucose)
De novo synthesis – make glucose from lactate, glycerol, amino acids
Predict the changes in blood glucose during the first few hours of a fast
Initial fall in BGL as tissues consume glucose → Liver responds by releasing glucose into bloodstream (glycogenolysis) → overall BGL stay constant
What is phosphorylase?
an enzyme that breaks glucose off glycogen
What is the process of the phosphorylation cascade?
Glucagon binds →
Adenyl cyclase makes cAMP →
cAMP activates PKA →
PKA phosphorylates phosphorylase kinase →
Phosphorylase kinase activates glycogen phosphorylase
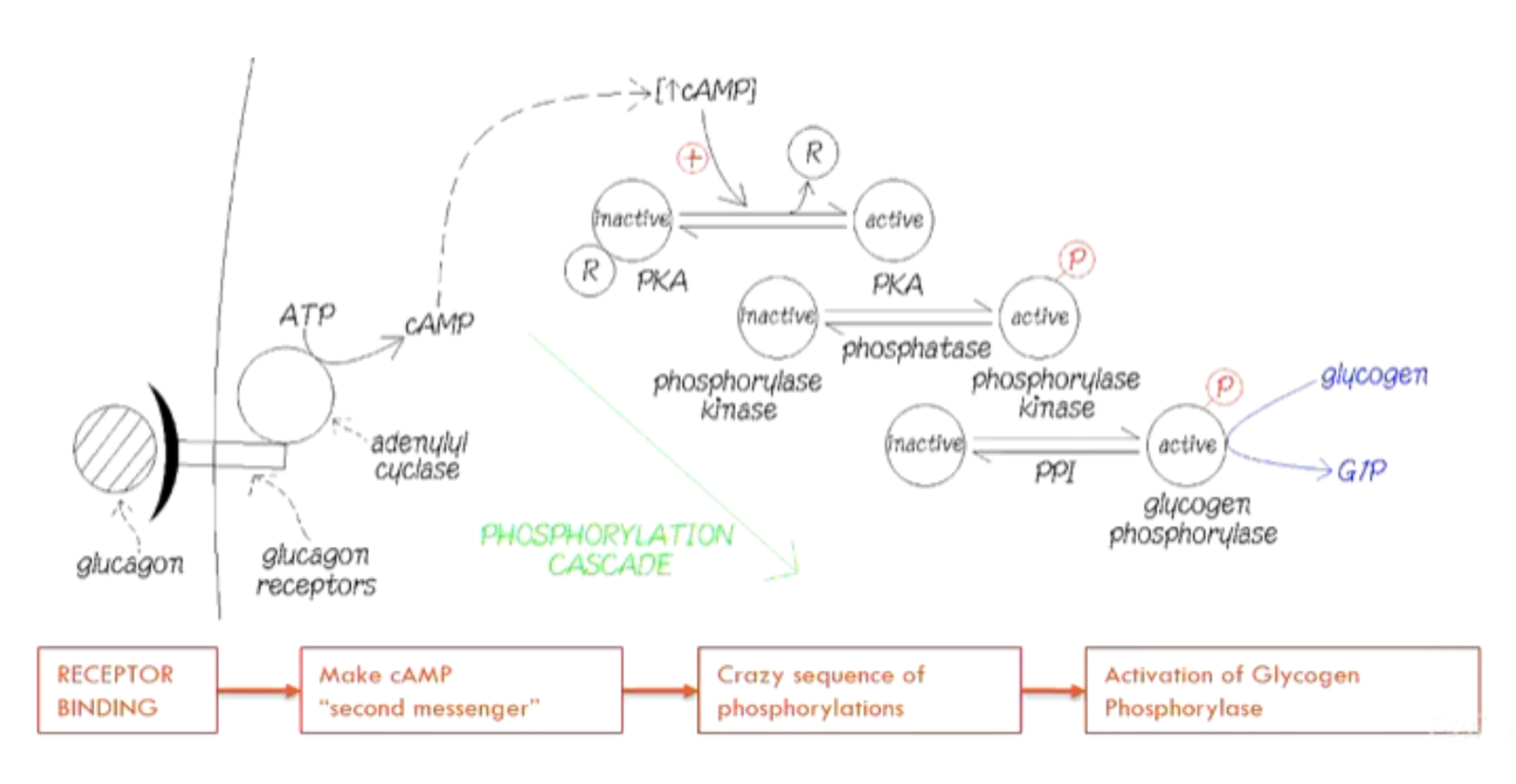
Why is this phosphorylation cascade better than direct binding?
Amplification through cAMP and cascade: Massive response from small signal
(Little ATP use, more control over the process)
Why doesn’t muscle break down much glycogen?
Muscle does NOT break down much glycogen:
No glucagon receptors
No G6Pase (can’t convert G6P → glucose)
How can muscle help with maintaining BGL?
some glucose released via debranching enzyme, also G6P → lactate if PDH inhibited
What happens to liver glycogen stores after 24 h of starvation?
Liver glycogen stores (100g) last <24 hours because of brain
Important to shift other tissues to fatty acid metabolism before liver glycogen is gone
What does PKA phosphorylate to prompt lipolysis?
Hormone sensitive lipase (HSL) → activates it
Perilipin (shell surrounding fat vacuole) → allows HSL to interact with fat → releases fatty acids into bloodstream
How is pyruvate dehydrogenase (PDH) regulated?
PDH regulated by phosphorylation:
Active when dephosphorylated
Inactive when phosphorylated byPDH kinase → stimulated by acetyl-CoA from FA oxidation) → Prevents oxidation of glucose during fasting
What is the glucose fatty acid cycle?
Fatty acid oxidation → ↑acetyl-CoA → stimulates PDH kinase → PDH phosphatase activity → inhibits PDH.
PDH inhibition → glucose not oxidised, converted to pyruvate, then lactate → Conserves glucose for brain
Summarise the patterns of fuel selection and mobilisation in early starvation
Early starvation: No dietary glucose → rely on stored fuels.
Liver glycogen → glucose
Lipolysis activated → FAs for muscles (sparing glucose)
Gluconeogenesis begins (lactate, glycerol, amino acids).
Cori cycle and glucose-fatty acid cycle help conserve glucose.
What are the 2 main glucose sources in early starvation?
Lactate provides glucose via gluconeogenesis (recycling)
Glycerol (via lipolysis) is the only de novo source of new glucose: ~30g/day
What stimulates proteolysis?
After a few hours of low BGL, insulin secretion stops → stimulates lipolysis and proteolysis
What is proteolysis?
Release of amino acids from tissues (mostly muscle) → used for gluconeogenesis
What are amino acids converted into for gluconeogenesis?
Alanine (from Pyruvate )
Glutamate (from Alpha-ketoglutarate)
Aspartate (from Oxaloacetate)
(These keto-acids are then used in gluconeogenesis)
What is the urea cycle and where does it occur?
Urea cycle (in liver only): amine groups are converted into urea
Synthesised from glutamate and aspartate → consumes a lot of ATP
What is the difference between Ketogenic and glucogenic amino acids?
Ketogenic AA backbone → only makes acetyl-CoA → CANNOT be made into glucose
Glucogenic AA backbone → made into pyruvate or Krebs Cycle intermediates → CAN be made into glucose
What happens to the lipolysis rate after 2-3 days of starvation?
After 2-3 days of starvation, the rate of lipolysis will be at a maximum: more FA in bloodstream than needed
How does ATP demand affect the rate of beta-oxidation in different body tissues?
Rate of beta oxidation in most tissues depends on ATP demand
Regeneration of CoA by Krebs cycle needed to keep FA oxidation → krebs cycle regulated by ATP demand
BUT liver can do beta-oxidation even if there is no extra demand for ATP (can regenerate coA from acetyl-coA)
How does the liver dispose of acetyl CoA in starvation compared to non-hepatic tissues?
Non-hepatic tissues: acetyl-CoA → krebs cycle
Liver: acetyl-CoA → ketone bodies
What is the pathway of ketone body formation?
2 acetyl-Coa → acetoacetate (ketone body)
How is acetoacetate taken up in the body?
Acetoacetate → beta-hydroxybutyrate
Both taken up into tissues (including brain - now only uses 30g glucose/day)
Converted back to acetyl-CoA → used in Krebs cycle, also inhibits PDH (prevents waste of glucose)
What are the inefficiencies with Ketone bodies?
Ketone bodies will be lost in urine, and can spontaneously decarboxylate
How does fuel use by the brain change during extended starvation?
Day 1-2: Brain using 120g glucose a day, losing >100g protein/day
Day 3-4: Ketone bodies lower brain’s need for glucose: losing 75g protein/day
Day 5: Brain uses 30g glucose/day
Why is glucagon important in starvation?
Increases glycogen breakdown → glucose
Decreases glycogen synthesis (less stored glucose)
Decreases glycolysis in liver (less glucose used as a fuel)
Increases gluconeogenesis in liver (synthesis of glucose)
Increases fatty acid mobilisation (less glucose used as a fuel)
Increases ketogenesis (alternate energy source for brain)
What does adenylate kinase do?
Adenylate kinase buffers ATP levels: 2ADP↔ATP+AMP
How would a 10% decrease in ATP affect AMP?
Adenine nucleotides have ADP and AMP in lower amounts so are more sensitive
10% drop in ATP can result in a 600% increase in AMP
Adenylate kinase amplifies the change
When will minor changes in substrate concentration affect the rate of reaction?
At high substrate concentrations, minor changes in substrate concentration will not affect rate of reaction (already working as fast as they can)
Only affect speed when close to Km - concentration when enzyme is catalysing reaction at half max reaction velocity
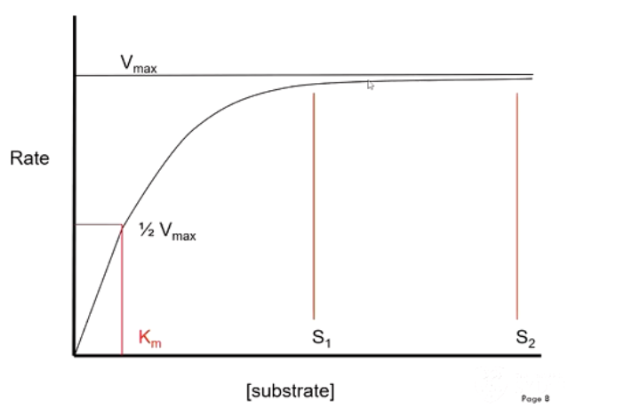
Describe the properties of rate limiting steps
Irreversible, saturated with substrate (working at Vmax)
Station at peak hour - gate becomes ‘saturated’ with people
increasing the number of people does not increase the rate.
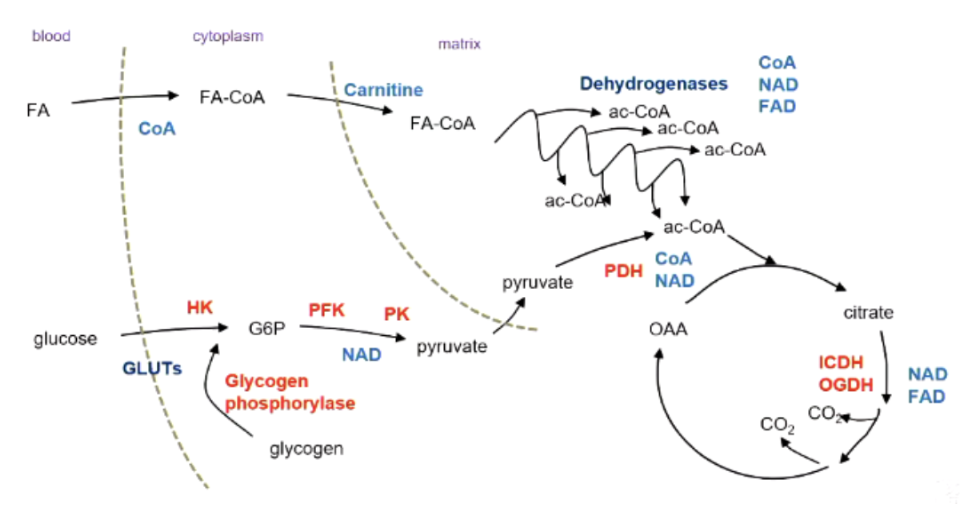
Rate limiting steps in fatty acid oxidation and glucose oxidation
Fatty acid oxidation - need CoA in cytoplasm, need carnitine to transport Fatty ac-coa into mitochondria, availability of CoA, NAD and FAD
Glucose oxidation - need GLUT transporters, hexokinase stops trapping glucose if we don’t use it, need phosphofructokinase, pyruvate kinase, and NAD+ for glycolysis to continue
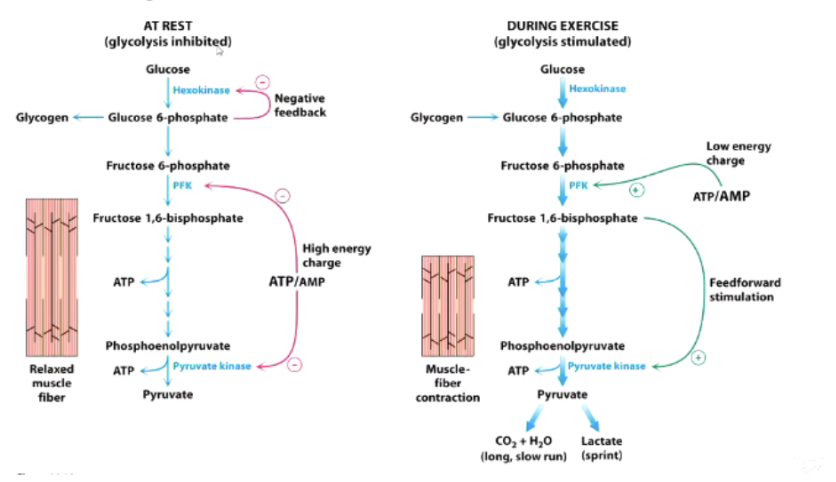
What inhibits and activates PFK?
Inhibited by:
High ATP (binds allosterically)
Citrate
Activated by:
AMP (relieves ATP inhibition)
What happens to glycolysis when resting vs exercising?
Glycolysis when resting - not performing much glycolysis so hexokinase inhibited, stop trapping glucose. Lots of ATP but not much AMP → switches off PFK
Glycolysis when exercising - using trapped glucose: inhibition of hexokinase relieved, trap more glucose. Now have low ATP and high AMP