VSEPR Theory Chart - Name Flashcards
0.0(0)
0.0(0)
Card Sorting
1/14
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
1
New cards
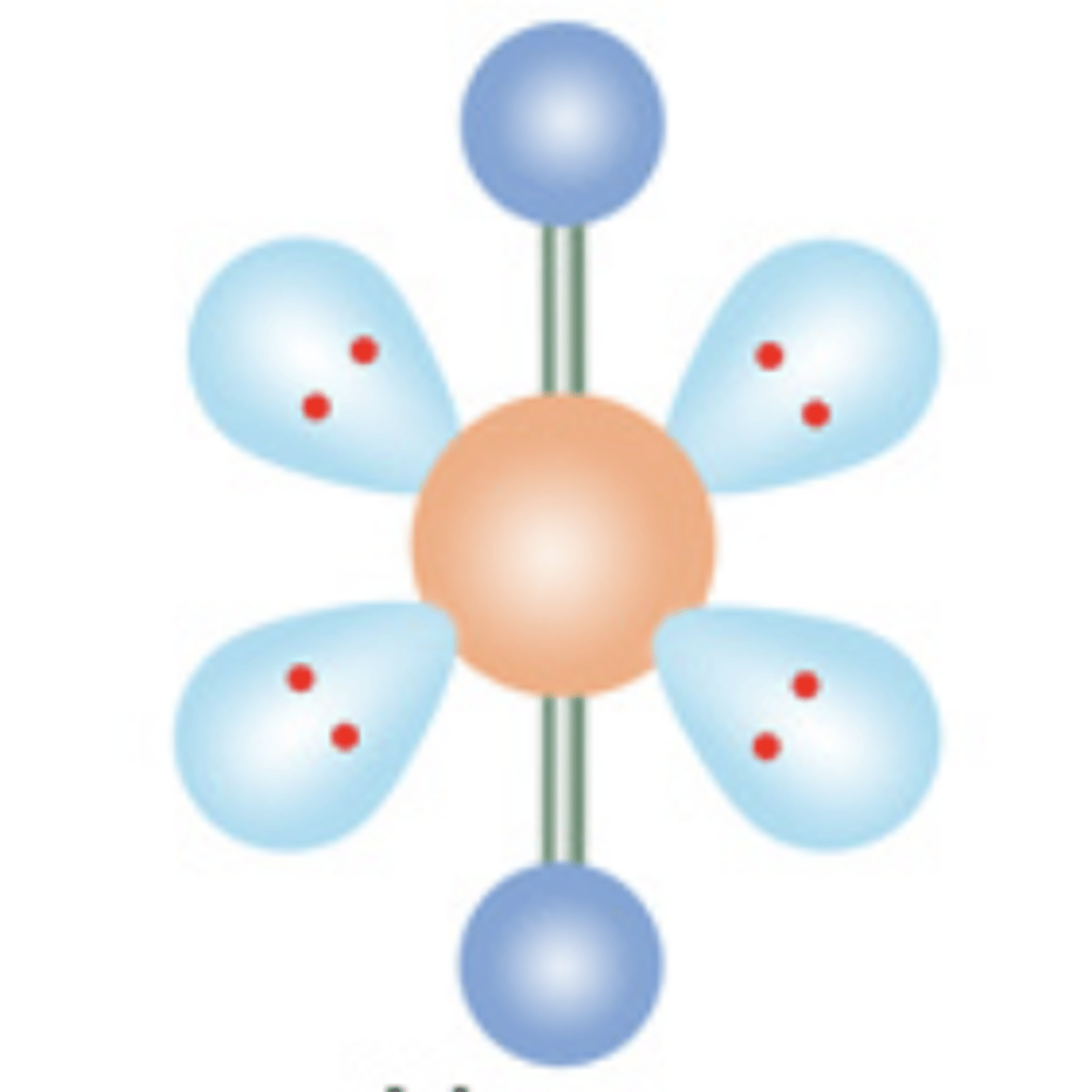
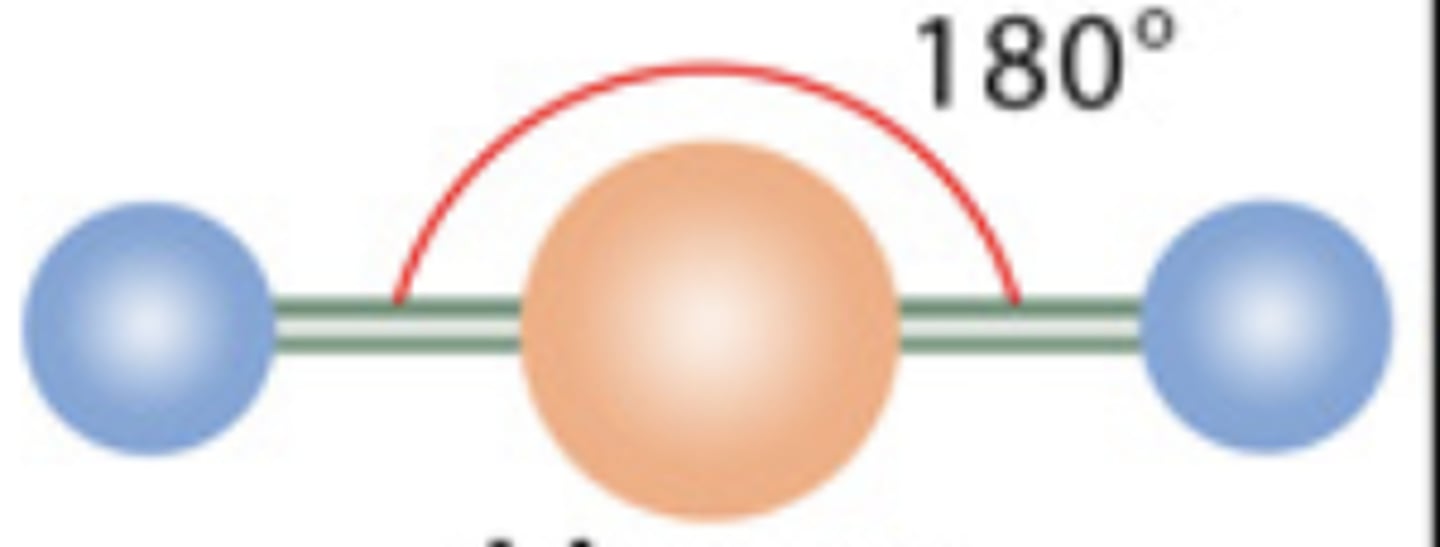
Linear
2 electrons; 0 lone pairs
2
New cards
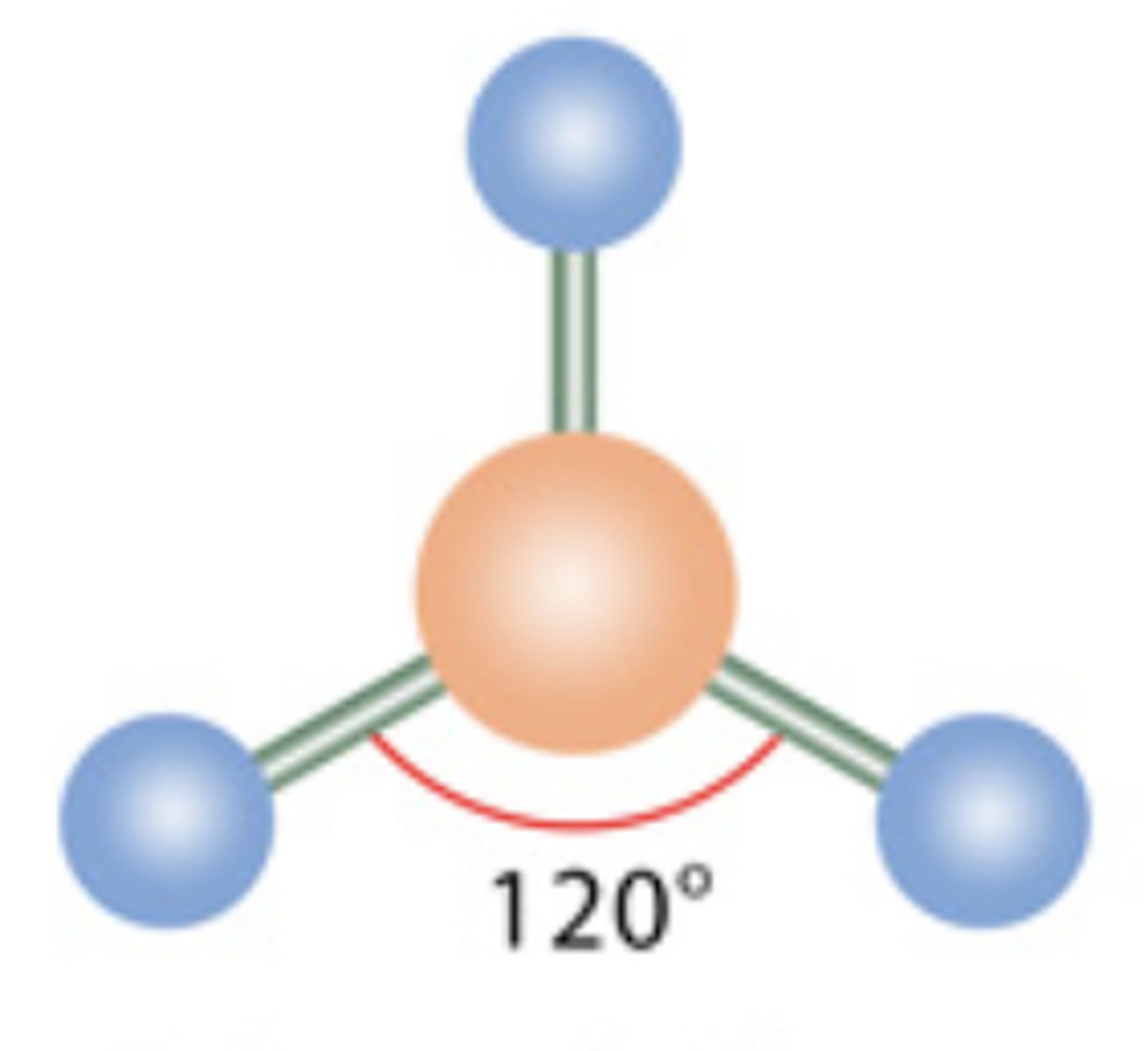
Trigonal Planar
3 electrons; 0 lone pairs
3
New cards
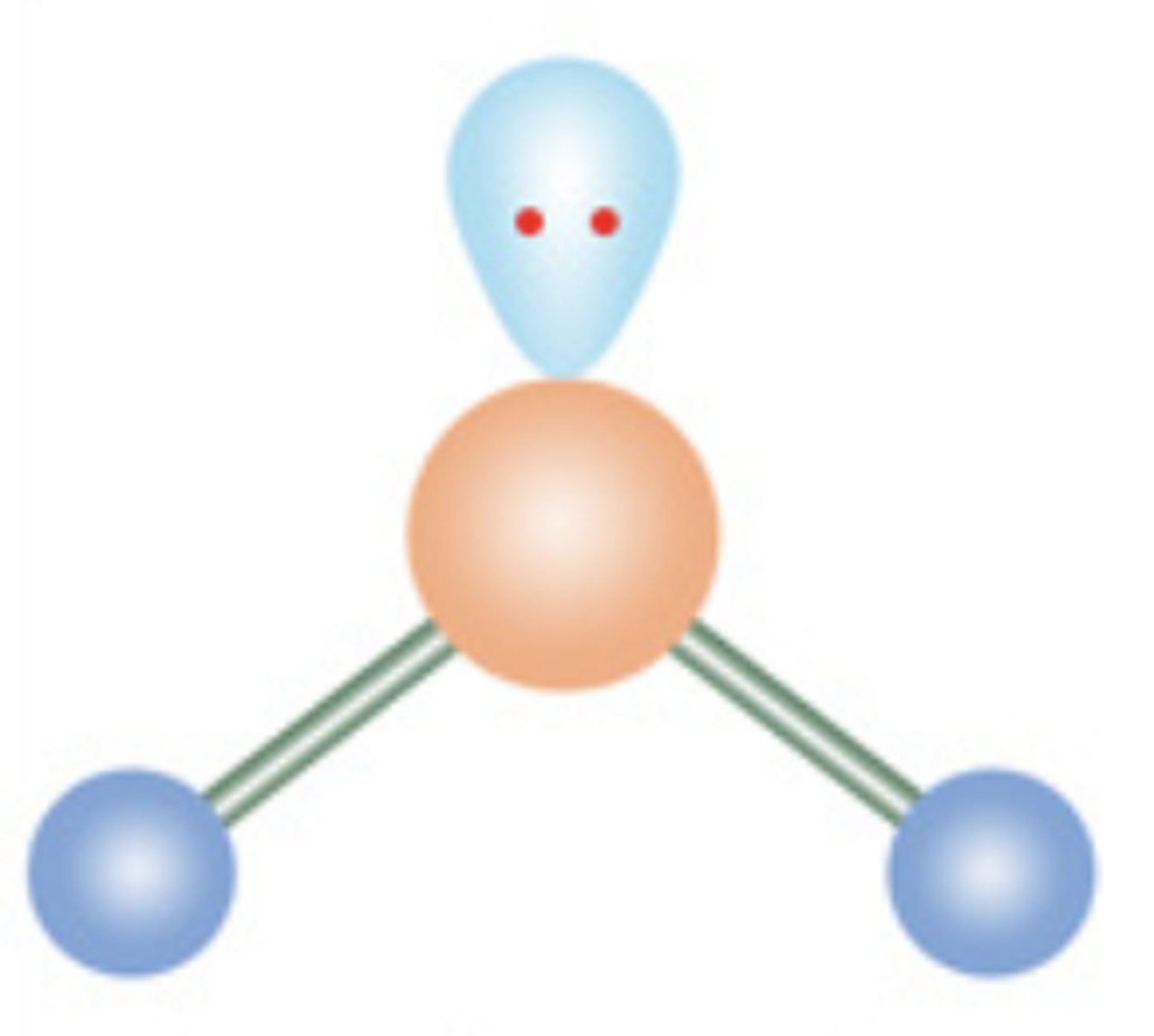
Angular or Bent
3 electrons; 1 lone pair
4
New cards
Tetrahedral
4 electrons; 0 lone pairs
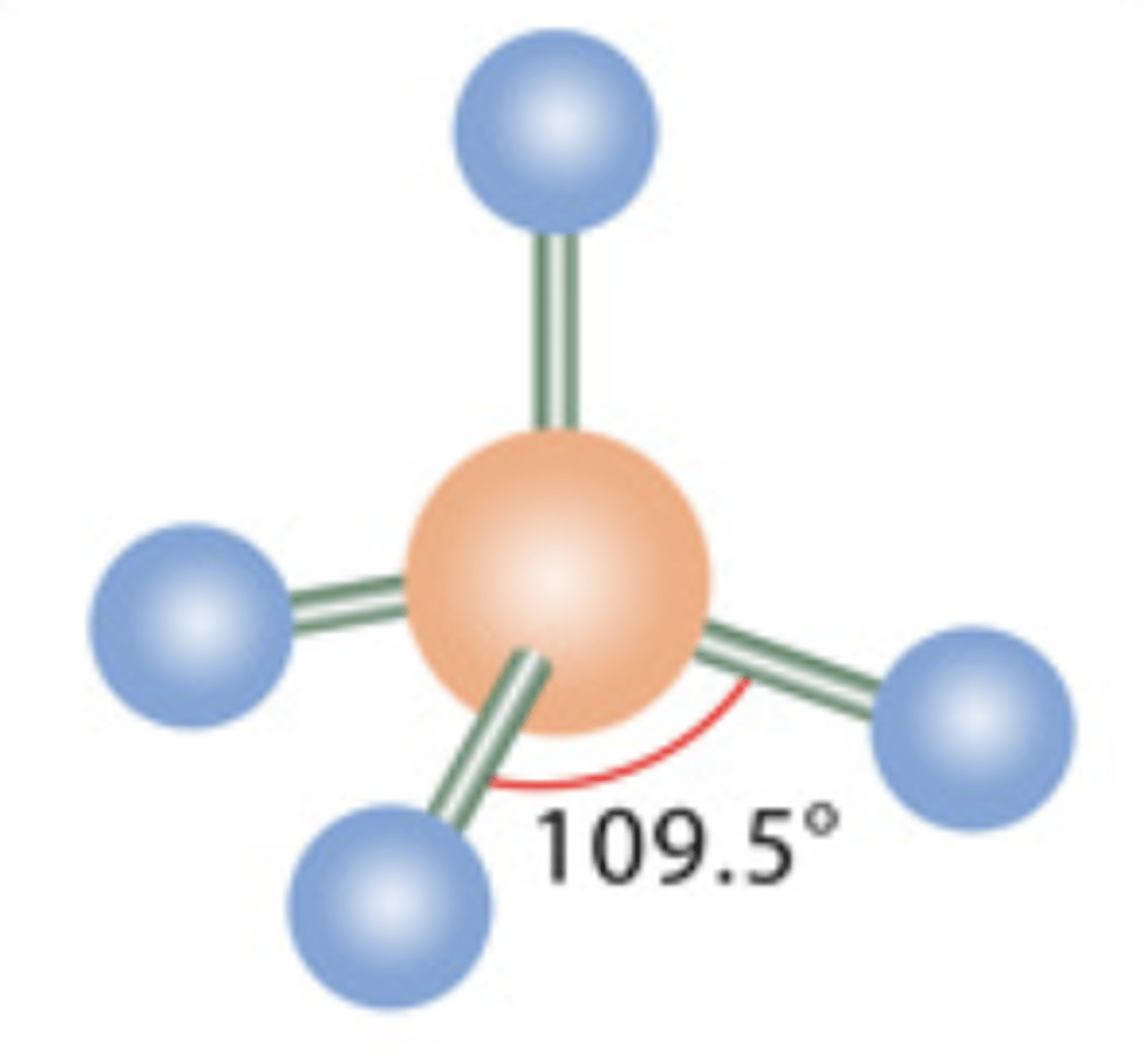
5
New cards
Trigonal Pyramidal
4 electrons; 1 lone pair
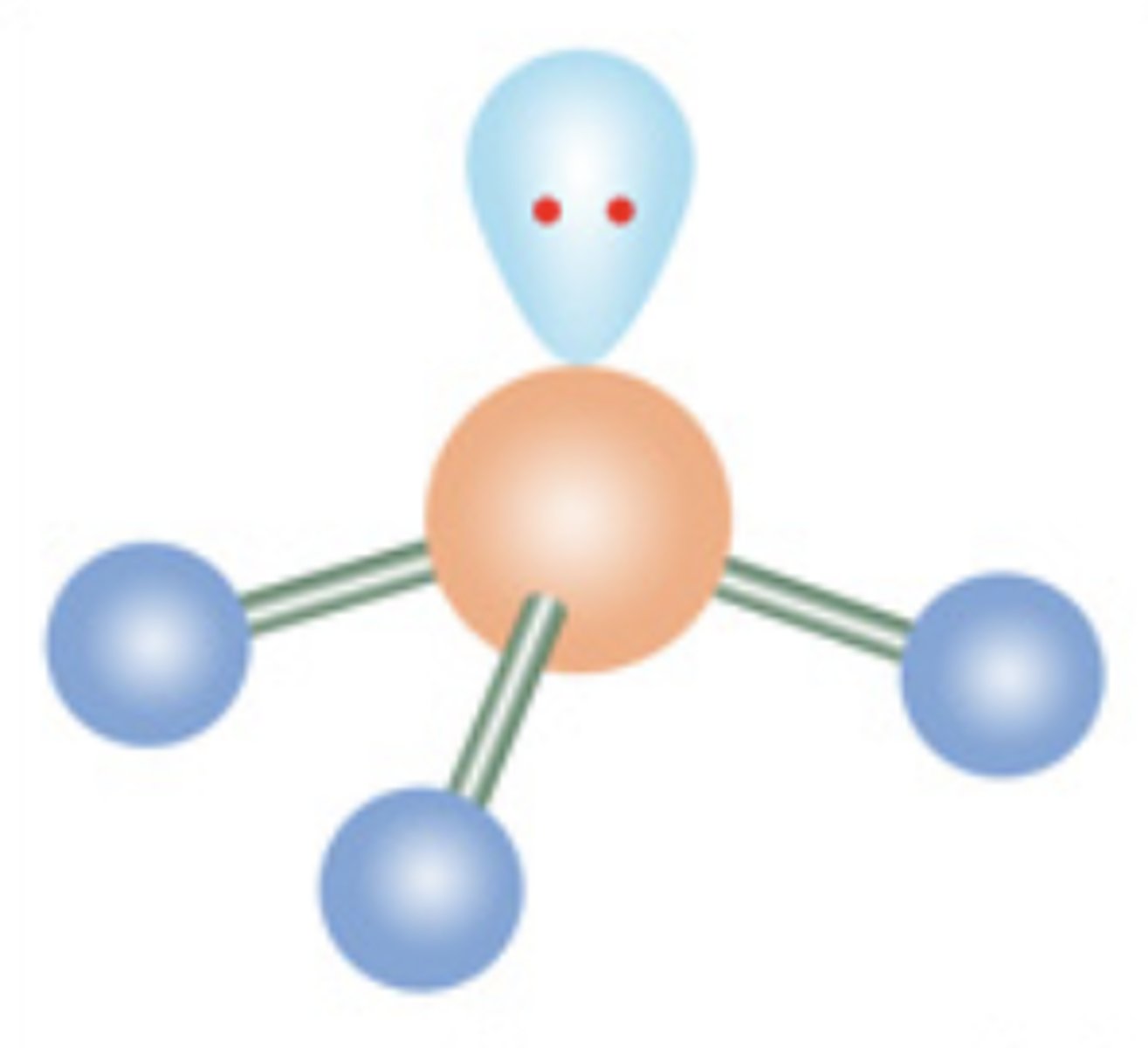
6
New cards
Angular or Bent
4 electrons; 2 lone pairs
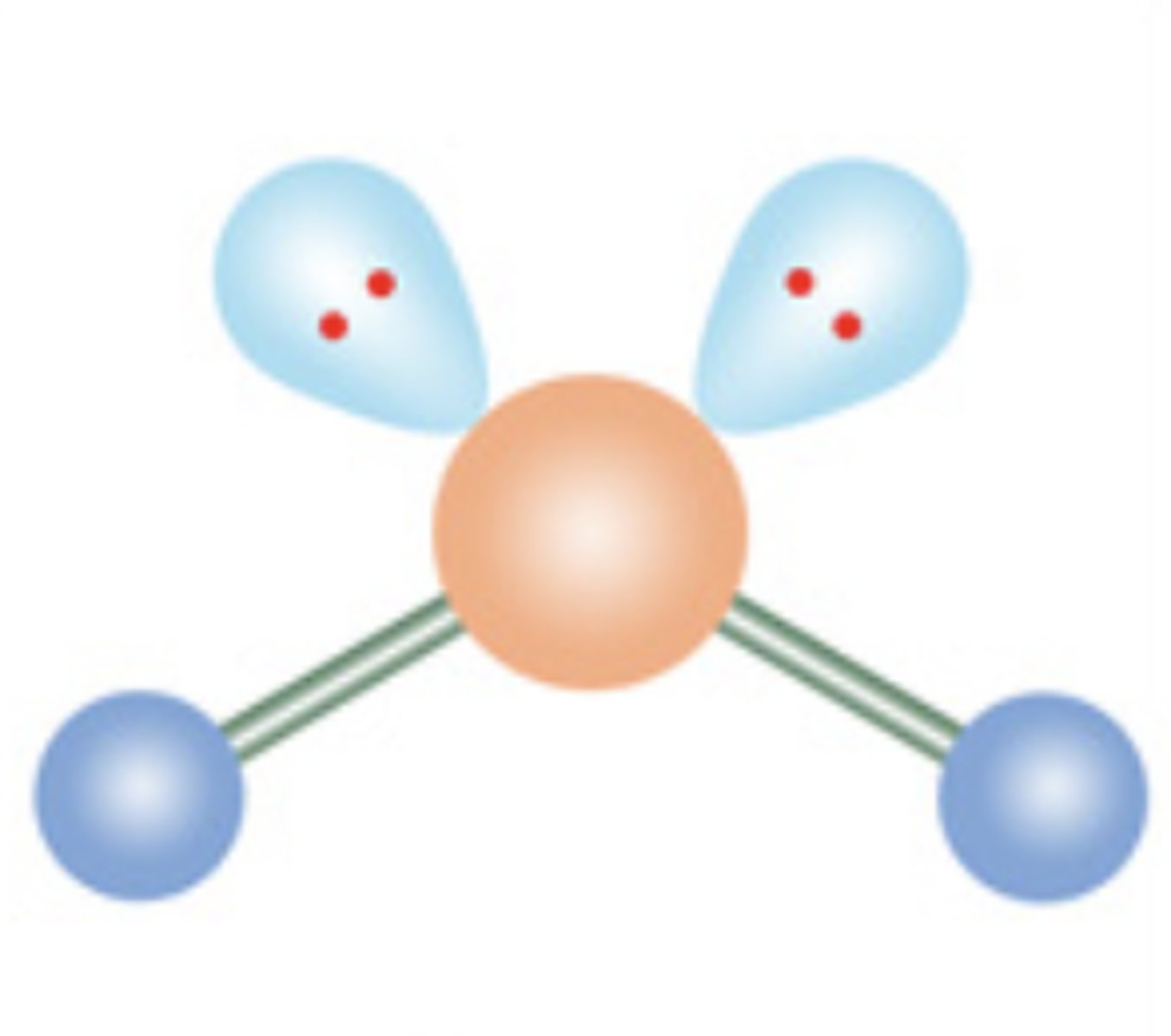
7
New cards
Trigonal Bipiramidal
5 electrons; 0 lone pairs
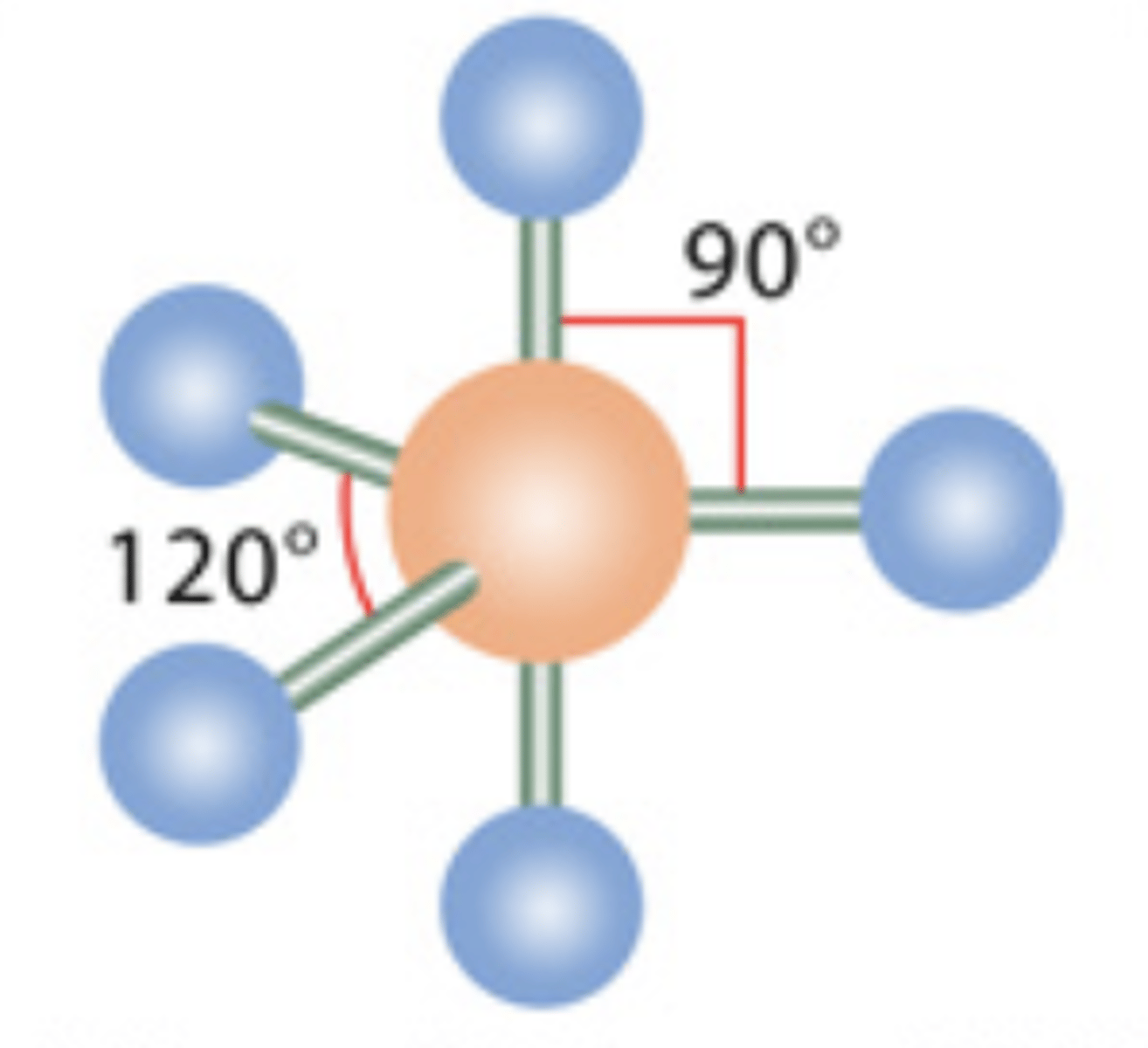
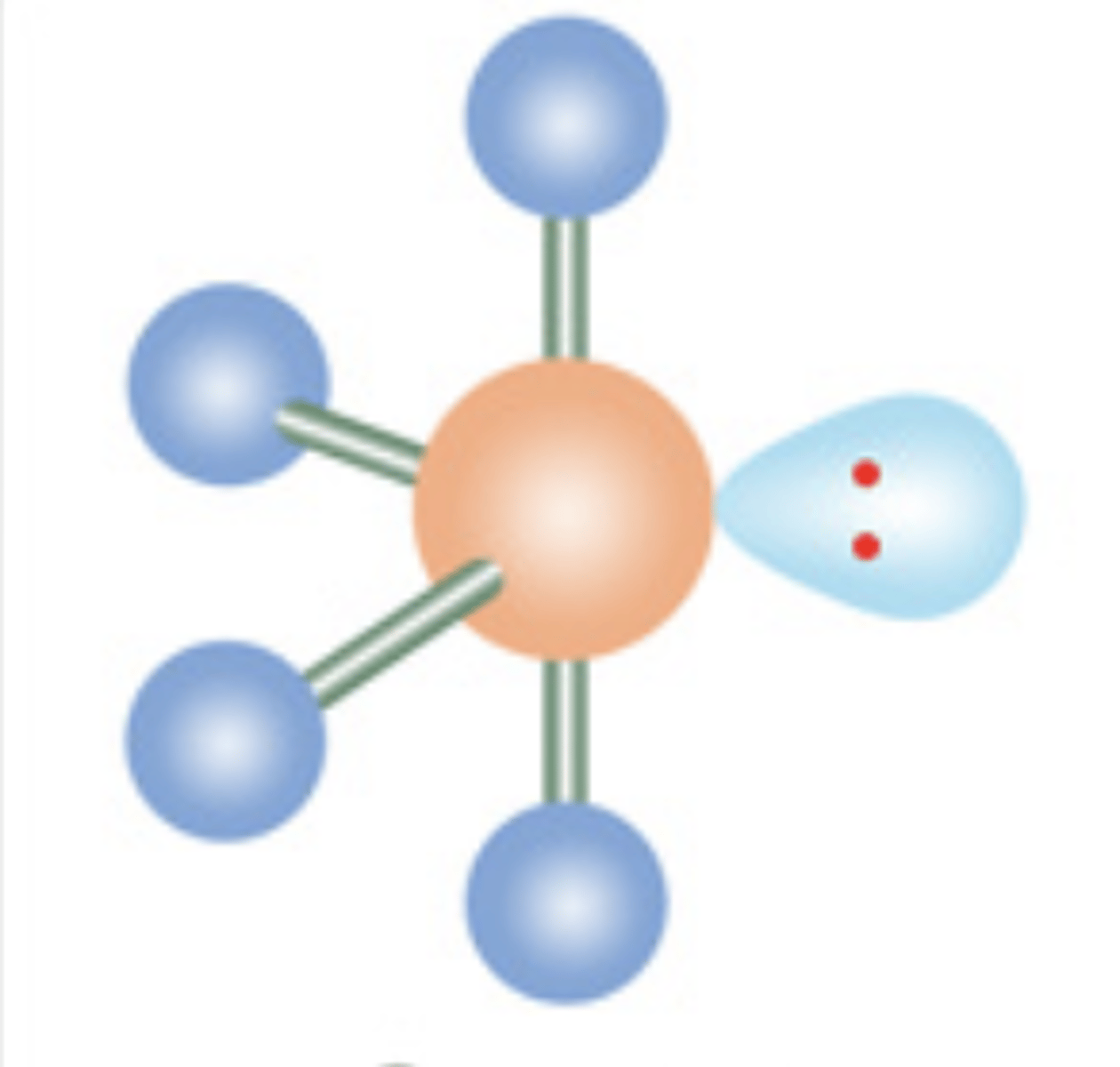
8
New cards
Seesaw
5 electrons; 1 lone pair
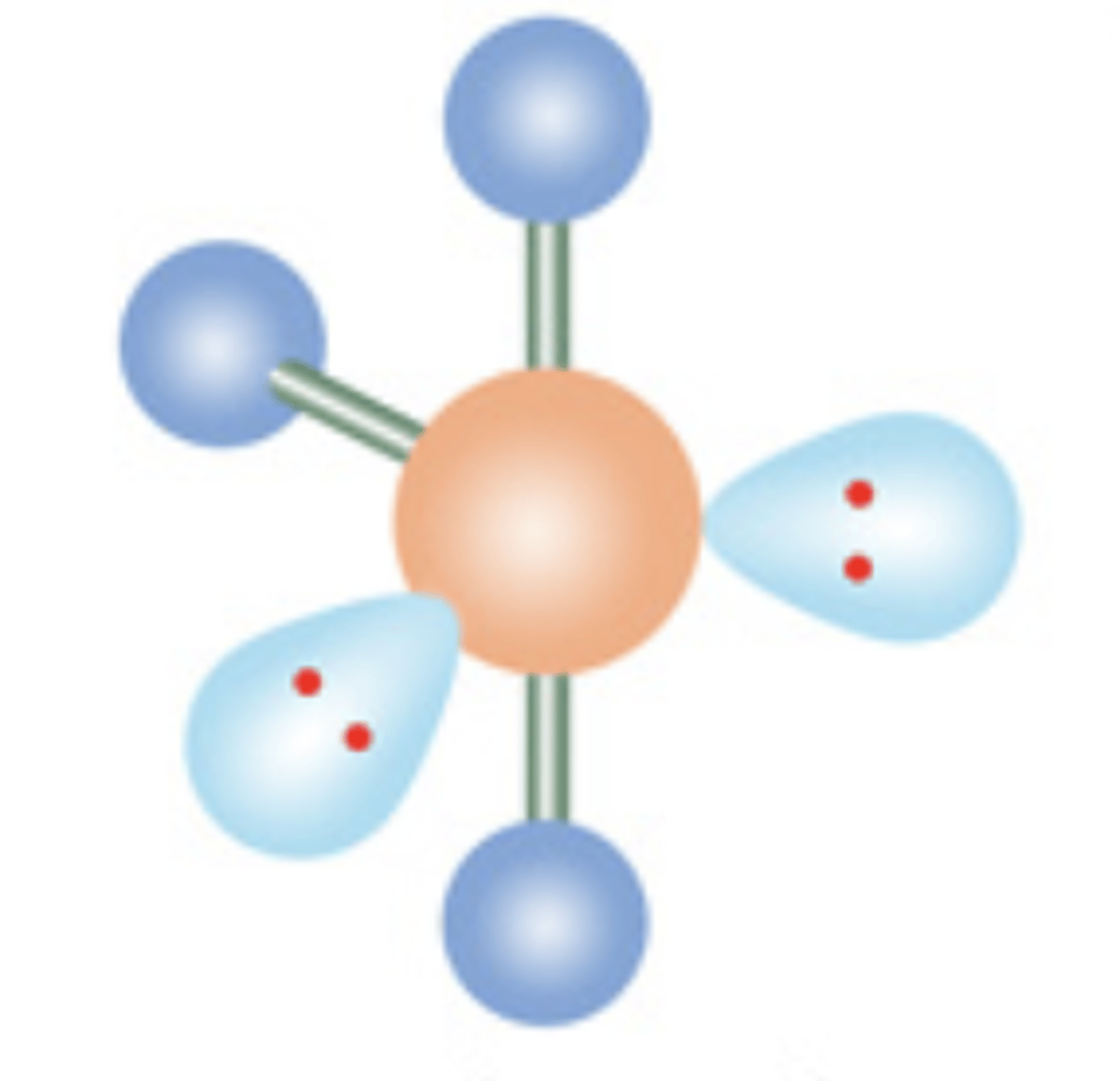
9
New cards
T-Shaped
5 electrons; 2 lone pairs
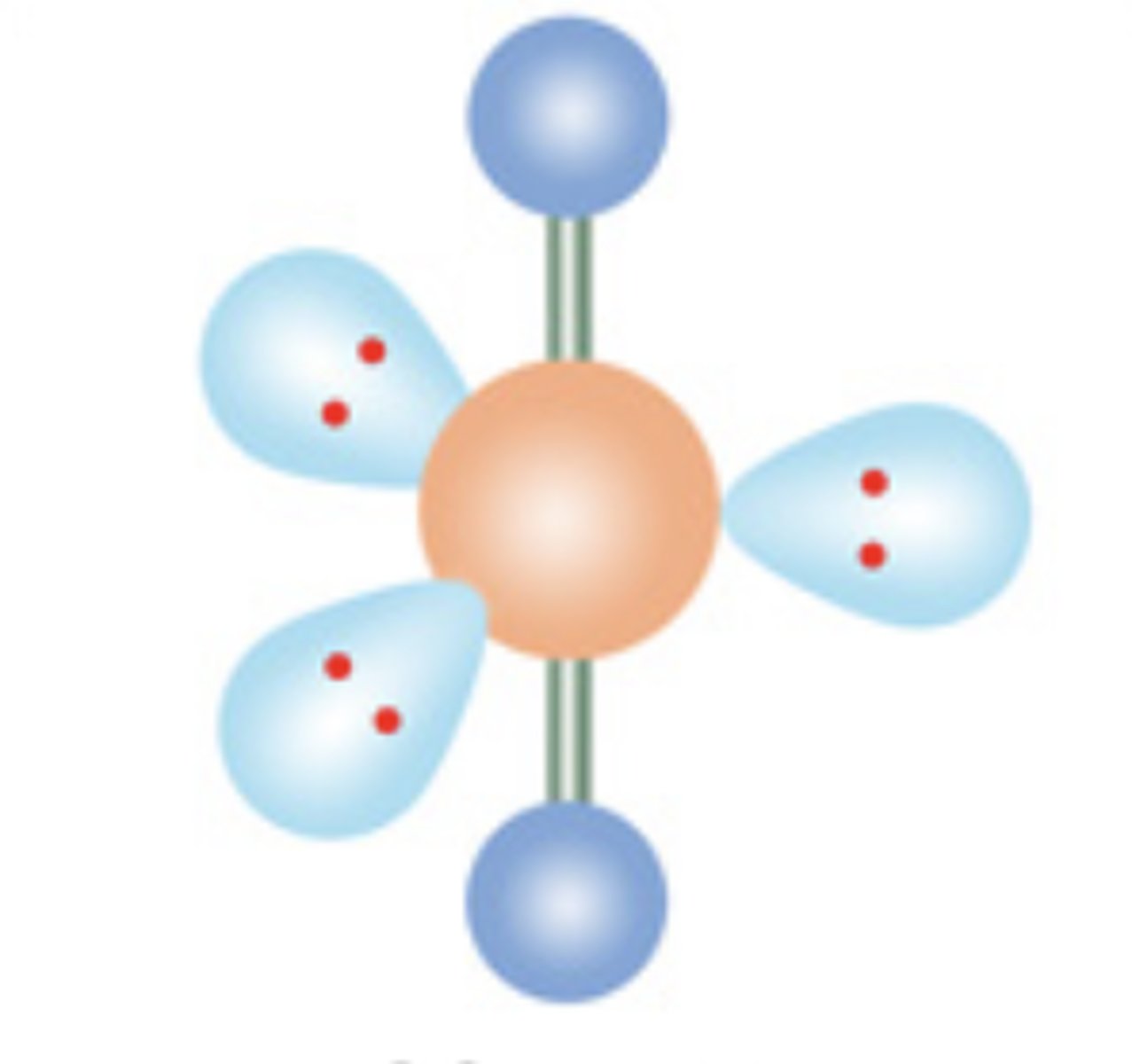
10
New cards
Linear
5 electrons; 3 lone pairs
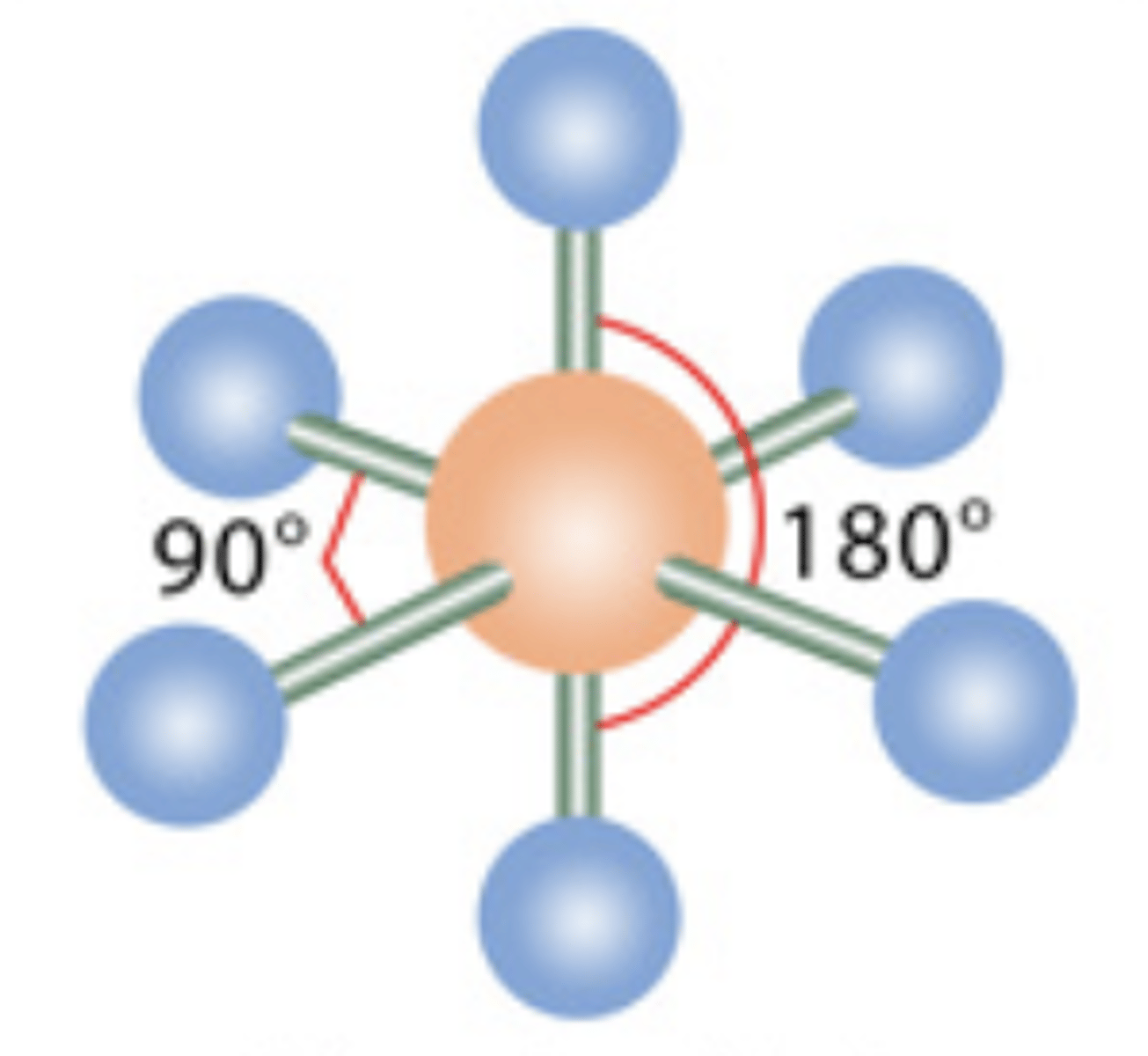
11
New cards
Octahedral
6 electrons; 0 lone pairs
12
New cards
Square Pyramidal
6 electrons; 1 lone pair
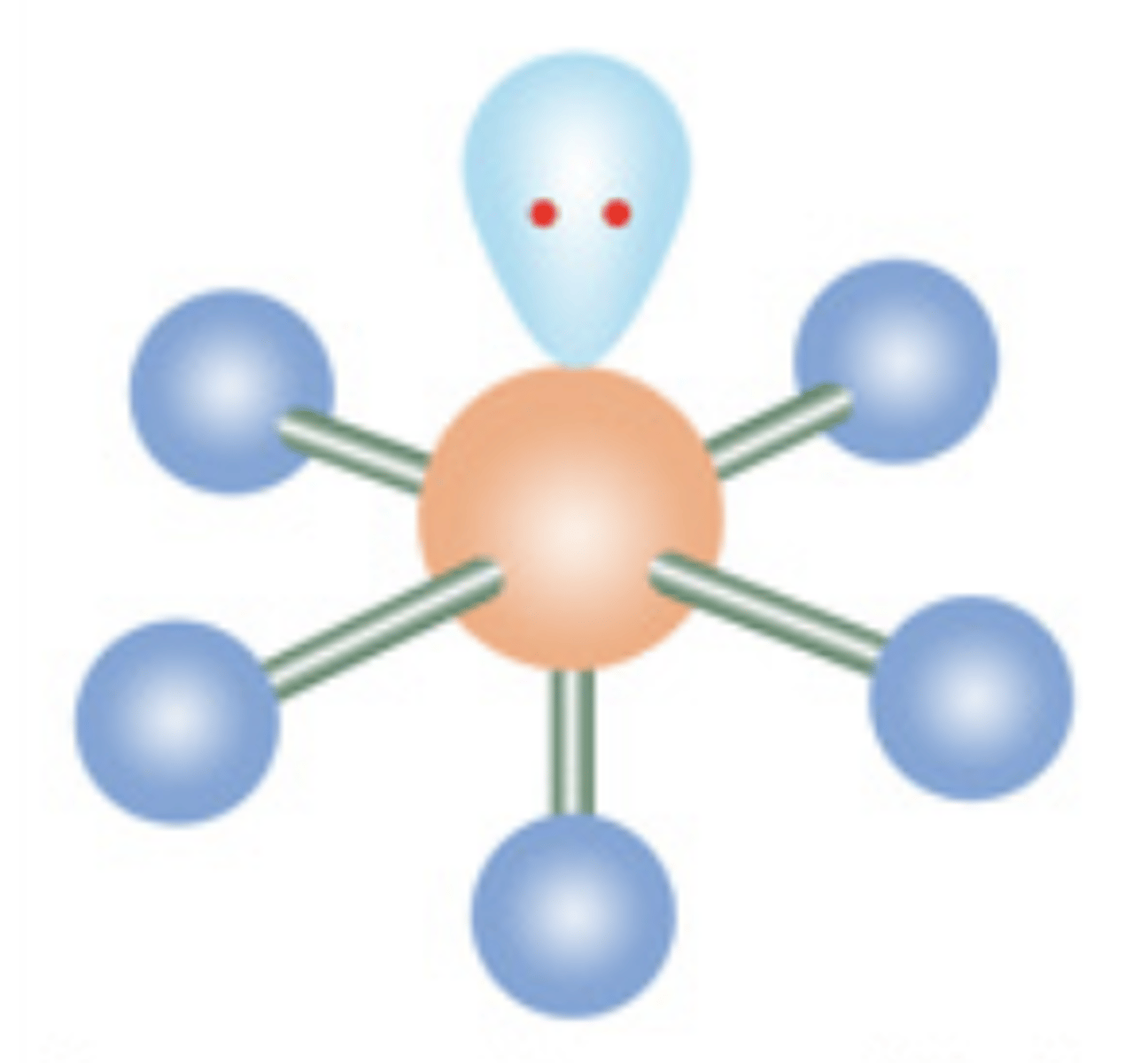
13
New cards
Square Planar
6 electrons; 2 lone pairs
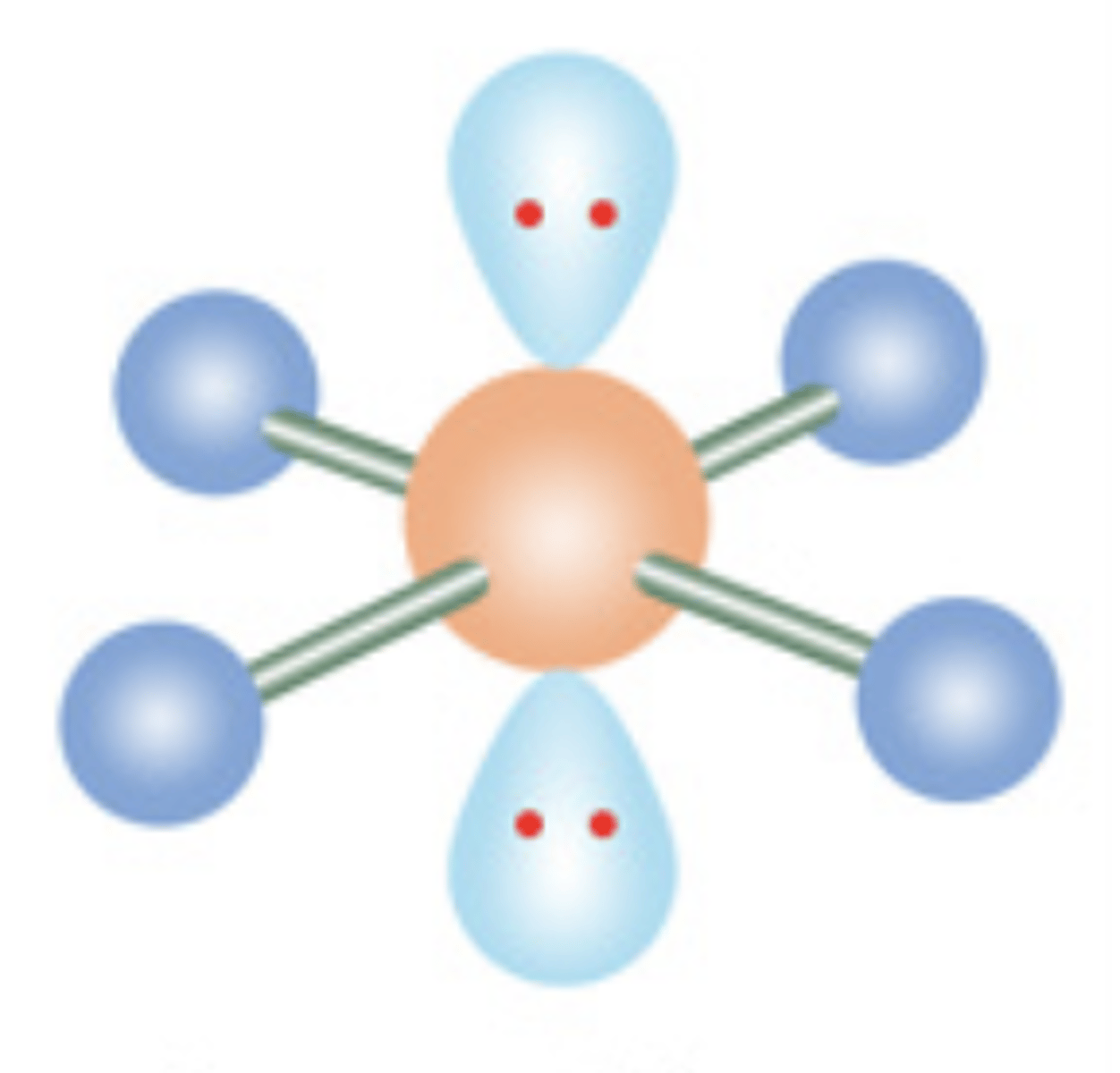
14
New cards
T-Shaped
6 electrons; 3 lone pairs
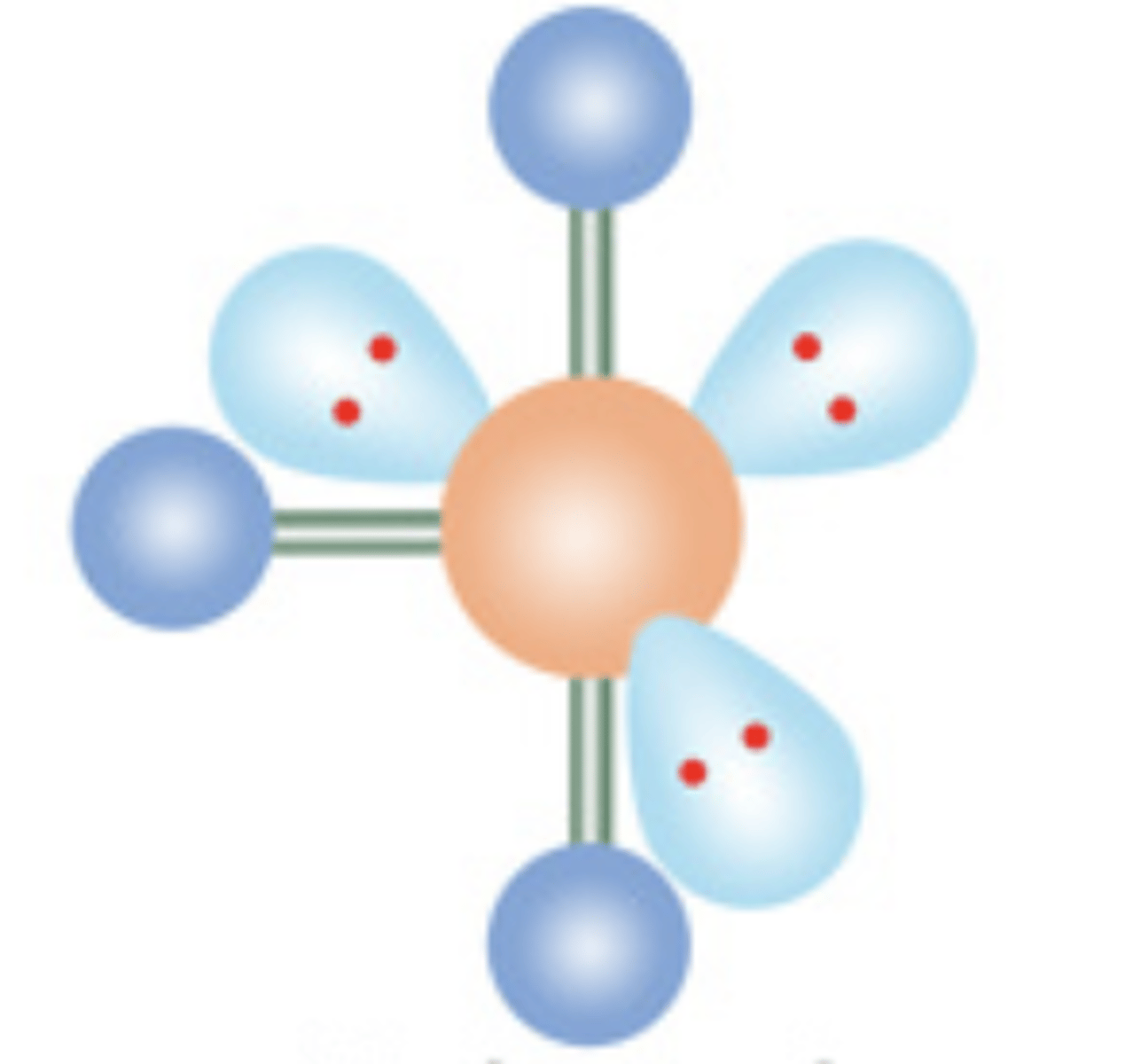
15
New cards
Linear
6 electrons; 4 lone pairs