CHEM 261 (Reaction Mechanisms)
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
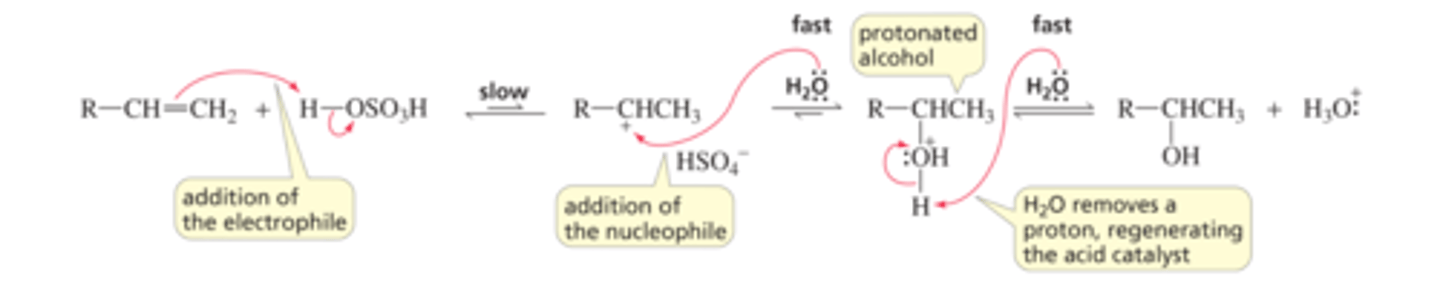
Mechanism for the Acid-Catalyzed Addition of water to an Alkene:
■ H + (an electrophile) adds to the sp2 carbon of the alkene (a nucleophile) that is bonded to the most hydrogens.
■ H 2O (a nucleophile) adds to the carbocation (an electrophile), forming a protonated alcohol.
■ The protonated alcohol loses a proton because the pH of the solution is greater than the pKa ofthe protonated alcohol (Section 2.10). (We saw that protonated alcohols are very strong acids;Section 2.6.
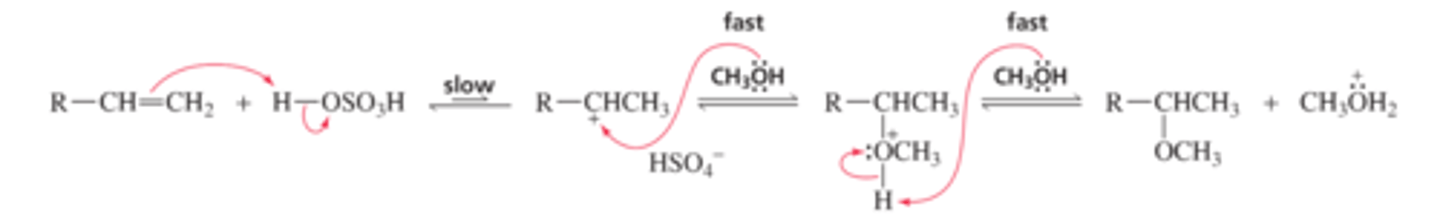
Mechanism for the Acid-Catalyzed Addition of an Alcohol to an Alkene
■ H+ (the electrophile) adds to the sp2 carbon bonded to the most hydrogens.
■ CH3OH (the nucleophile) adds to the carbocation, forming a protonated ether.
■ The protonated ether loses a proton, because the pH of the solution is greater than the pKa of the protonated ether (pKa ∼ -3.6).
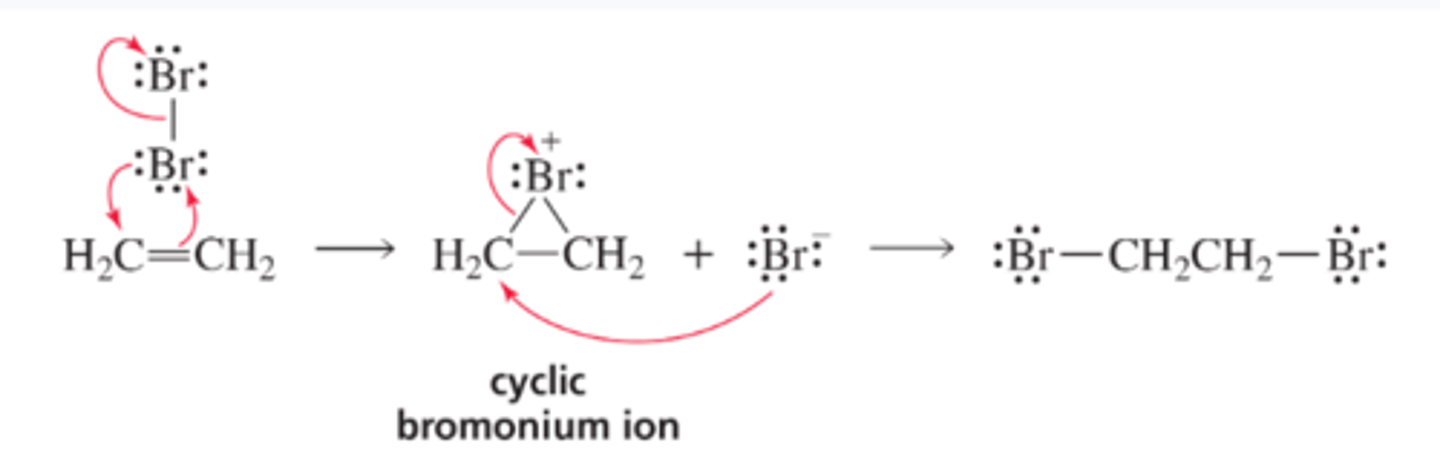
Mechanism for the Addition of Bromine to an Alkene
■ As the p electrons of the alkene approach a molecule of Br2, one of the bromines accepts those electrons and releases the shared electrons to the other bromine, which leaves as a bromide ion. Because bromine's electron cloud is close enough to the other sp2 carbon to form a bond, a cyclic bromonium ion intermediate is formed rather than a carbocation intermediate.
■ The cyclic bromonium ion intermediate is unstable because of the strain in the three-membered ring and the positively charged bromine, which withdraws electrons strongly from the ring carbons (Figure 6.4). Therefore, the cyclic bromonium ion reacts rapidly with a nucleophile (Br - ).
■ The mechanism for the addition of Cl2 is the same as the mechanism for the addition of Br2.
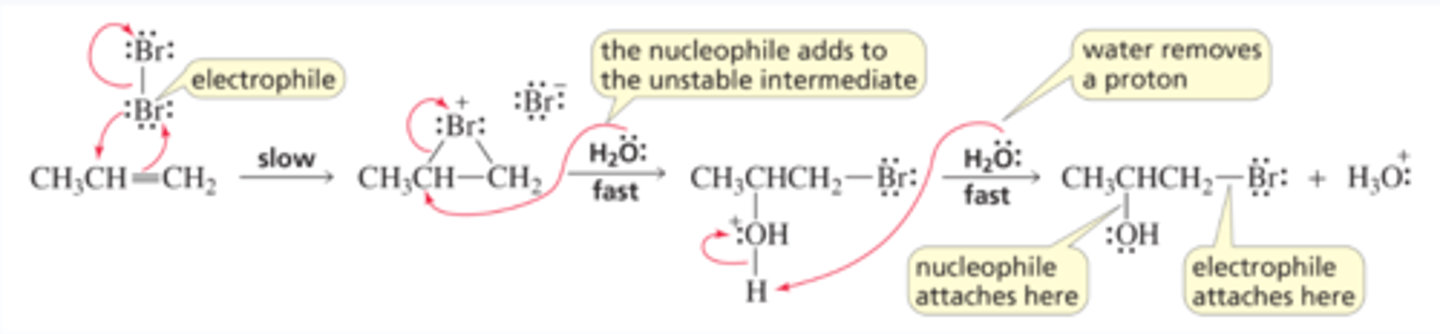
Mechanism for Halohydrin Formation
■ A cyclic bromonium ion (or chloronium ion) intermediate is formed in the first step because Br+ (or Cl+) is the only electrophile in the reaction mixture.
■ The relatively unstable cyclic bromonium ion intermediate rapidly reacts with any nucleophile it collides with. Two nucleophiles—H2O and Br -—are present in the solution, but the concentration of H2O far exceeds that of Br - . Consequently, the bromonium ion is much more likely to collide with water than with Br - .
■ The protonated halohydrin is a strong acid (Section 2.3), so it readily loses a proton
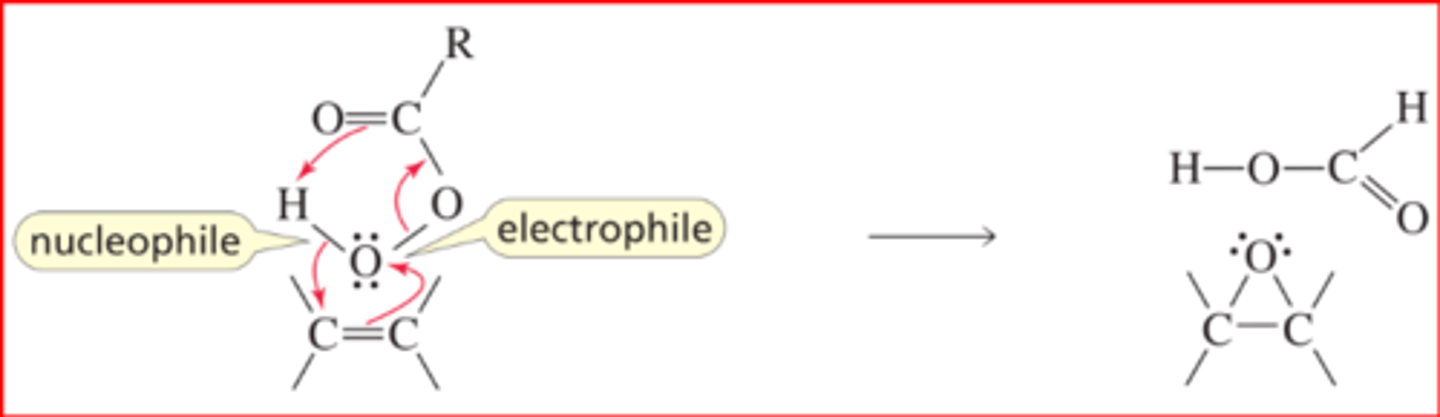
Mechanism for the Epoxidation of an Alkene
■ An oxygen atom of the OOH group (the O that is attached to the H) of the peroxyacid is electron-deficient and is, therefore, an electrophile. It accepts the electrons from the p bond of the alkene, which causes the weak O¬O bond of the peroxyacid to break.
■ The electrons from the O¬O bond are delocalized, causing the π bond of the C"O group to break and pick up a proton (Section 2.8).
■ As the O¬H bond breaks, the bonding electrons (the nucleophile) add to the other sp2 carbon of the alkene.
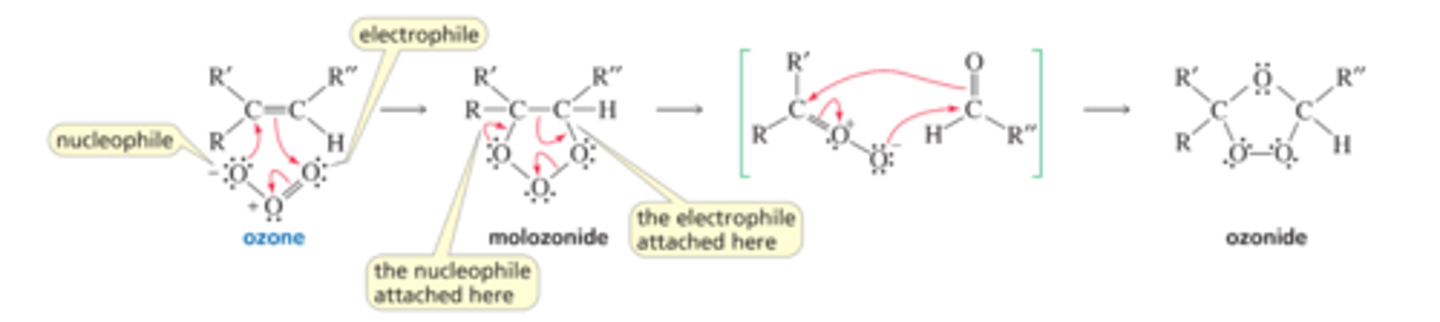
Mechanism of Ozonide Formation
■ The electrophile (the doubly bonded oxygen at one end of the ozone molecule) adds to one of the sp2 carbons, and a nucleophile (the negatively charged oxygen at the other end) adds to the other sp2 carbon. The product is a molozonide.
■ The molozonide is unstable because it has two O¬O bonds; it immediately rearranges to a more stable ozonide
Products of Ozonide
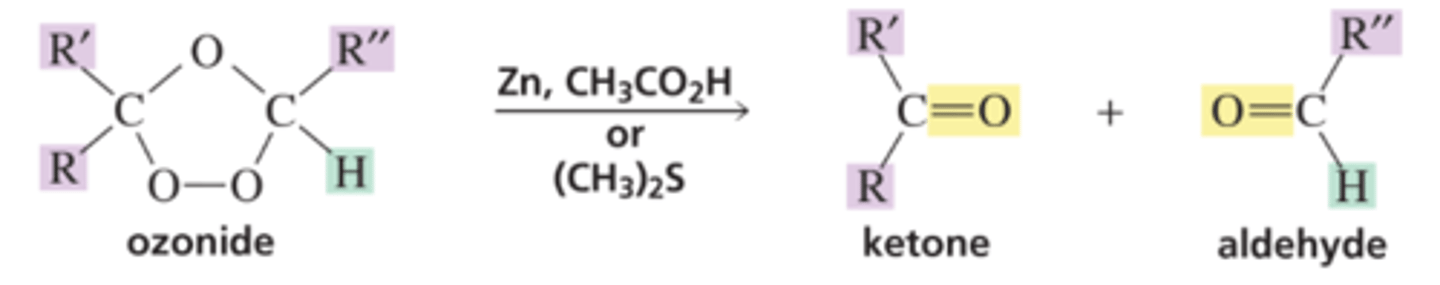
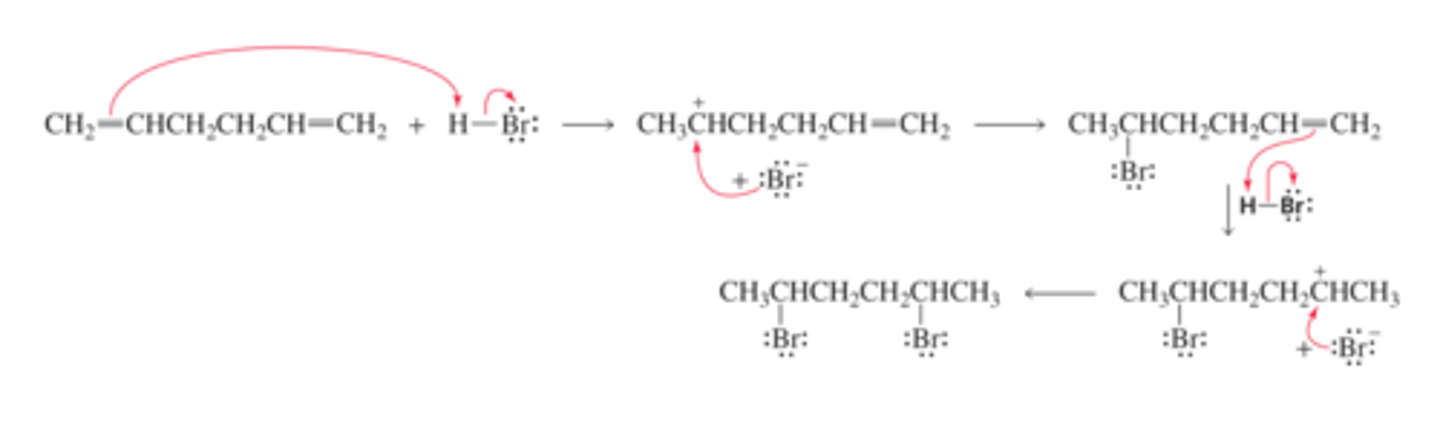
Mechanism for the Reaction of an Isolated Diene with Excess HBr
■ The electrophile (H+) adds to the sp2 carbon bonded to the most hydrogens in order to form the more stable carbocation (Section 6.4).
■ The bromide ion adds to the carbocation.
■ Because there is an excess of the electrophilic reagent, there is enough reagent to add to the other double bond; again the H+ adds to the sp2 carbon bonded to the most hydrogens.
■ The bromide ion adds to the carbocation.
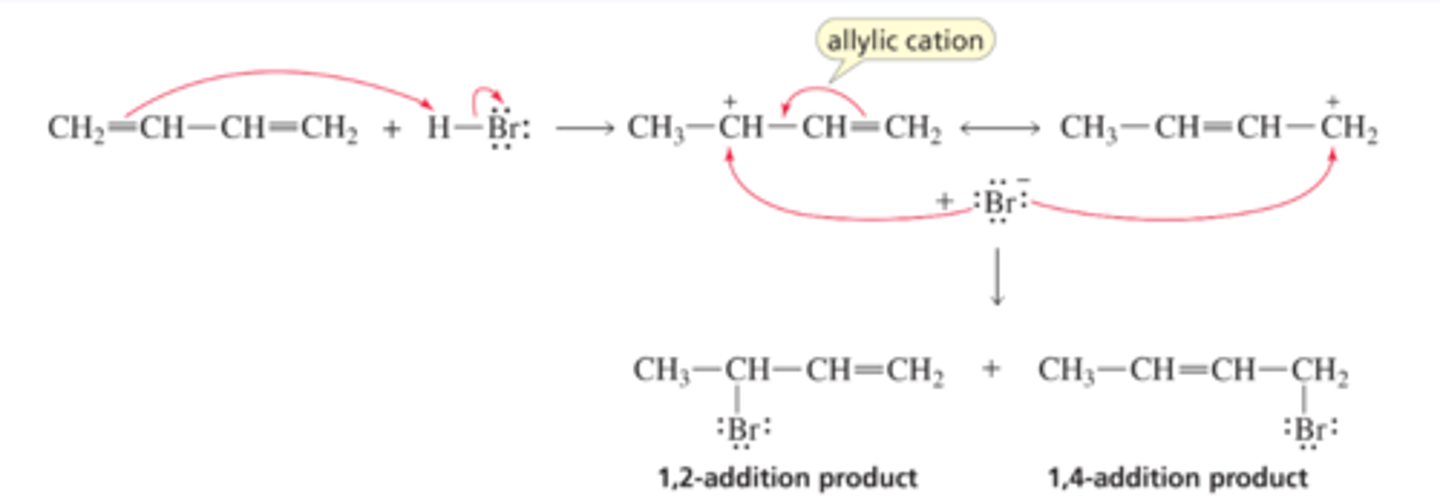
Mechanism for the Reaction of a Conjugated Diene with HBr
■ The proton adds to C-1, forming an allylic cation. The allylic cation has delocalized electrons.
■ The resonance contributors of the allylic cation show that the positive charge is shared by C-2 and C-4. Consequently, the bromide ion can add to either C-2 or C-4 to form the 1,2-addition product or the 1,4-addition product, respectively
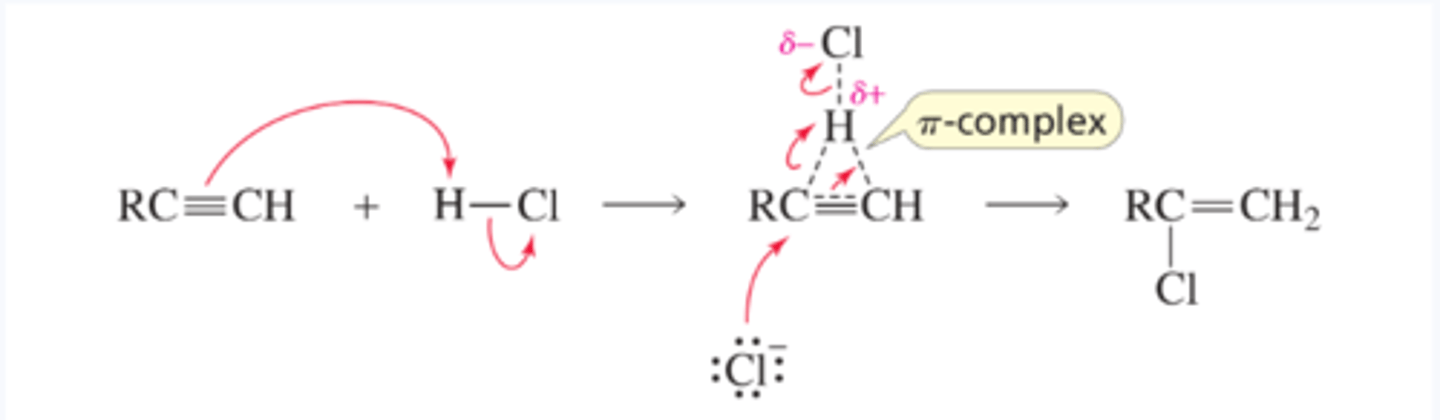
Mechanism for Electrophilic Addition of a Hydrogen Halide to an Alkyne
■ The alkyne (a nucleophile) reacts with an electrophile to form a p-complex.
■ Chloride ion adds to the p-complex, forming a halo-substituted alkene.
Addition of Water to Various Alkynes
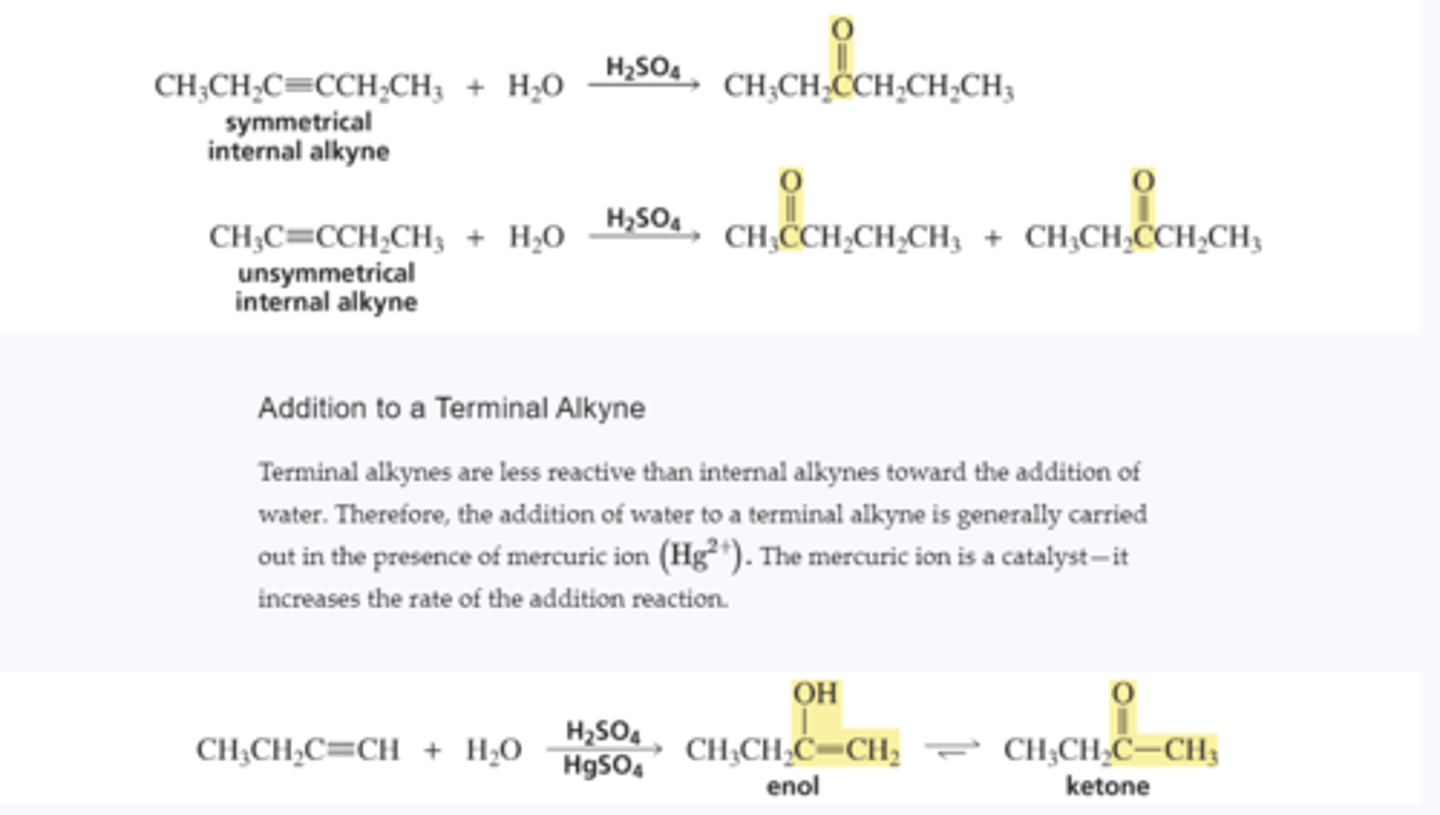
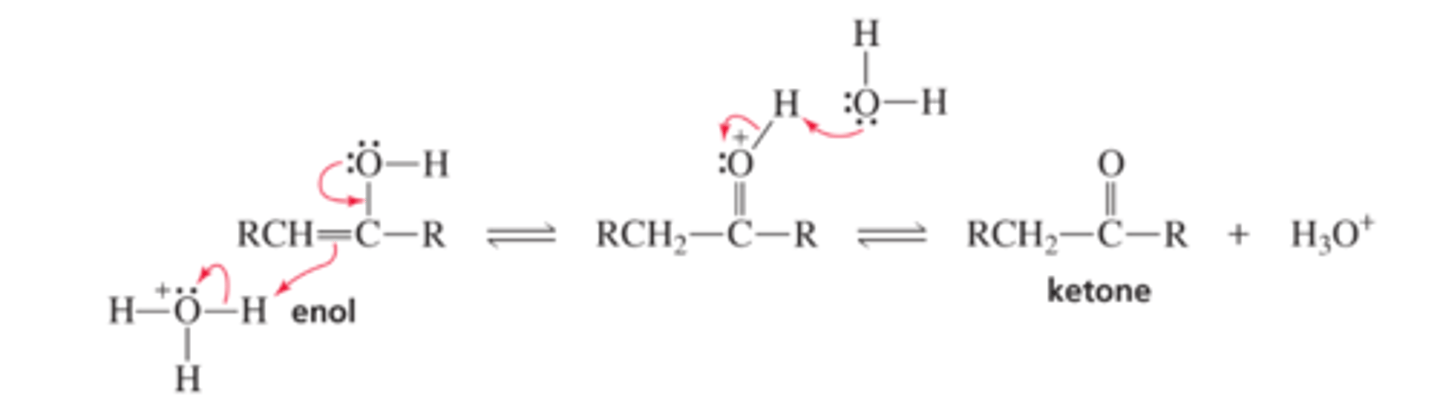
Mechanism for Acid-Catalyzed Keto-Enol Interconversion
■ A p bond forms between carbon and oxygen and, as the p bond between the two carbons breaks, carbon picks up a proton.
■ Water removes a proton from the protonated carbonyl group
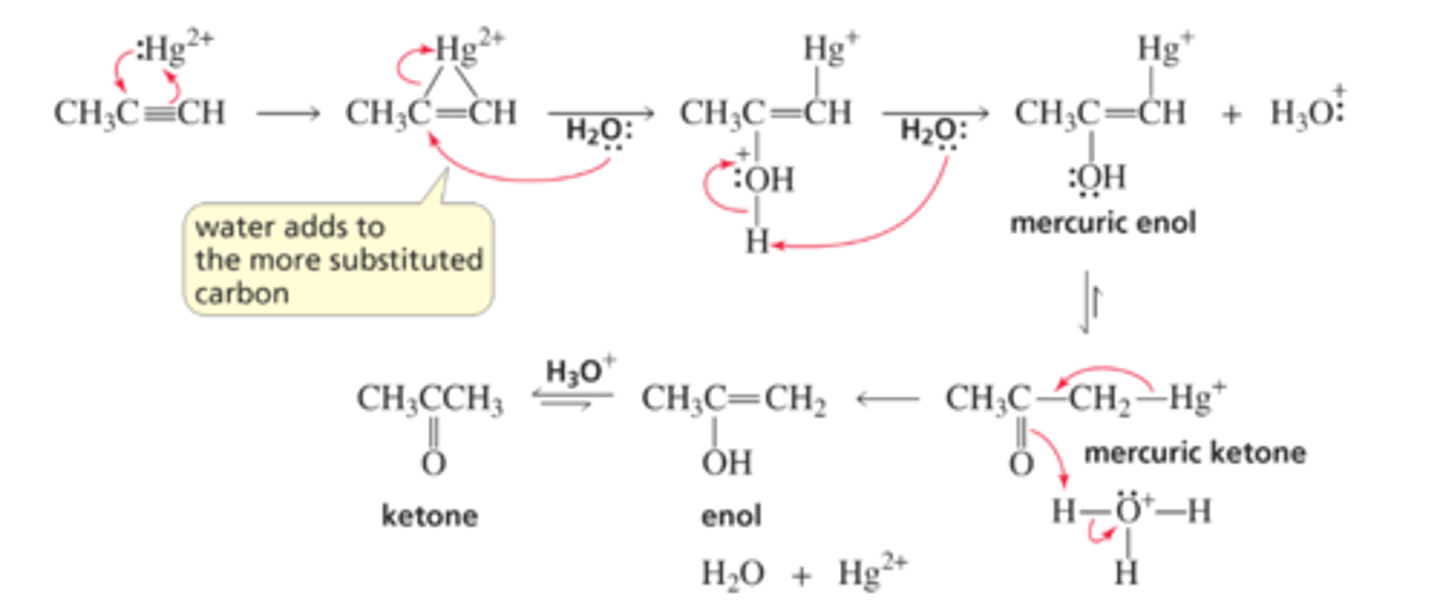
Mechanism for the Mercuric-Ion-Catalyzed Hydration of an Alkyne
■ Reaction of the alkyne with mercuric ion forms a cyclic mercurinium ion. (Two of the electrons in mercury's filled 5d atomic orbitals are shown.)
■ Water (the nucleophile) adds to the more substituted carbon of the cyclic intermediate (Section 6.9).
■ The protonated OH group loses a proton to form a mercuric enol, which immediately tautomerizes to a mercuric ketone.
■ Loss of the mercuric ion forms an enol, which tautomerizes to a ketone in an acid-catalyzed reaction (see page 300).
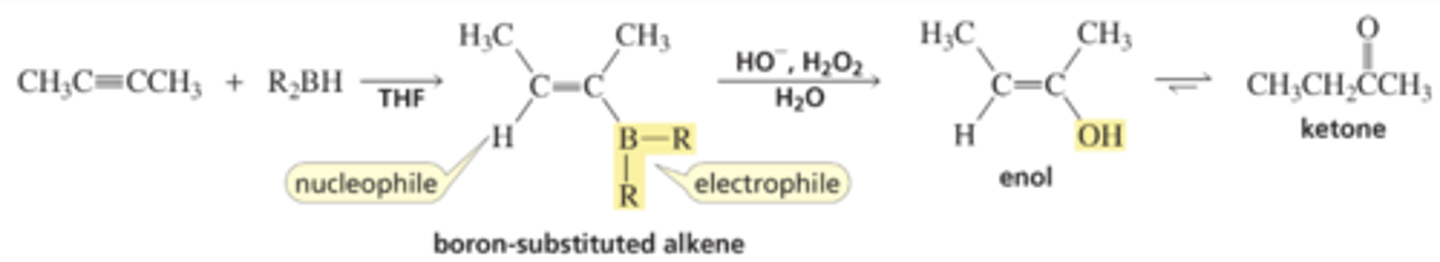
Addition of Borane to an Alkyne: Hydroboration-Oxidation
Alkyne reacts with H2 Pd/C
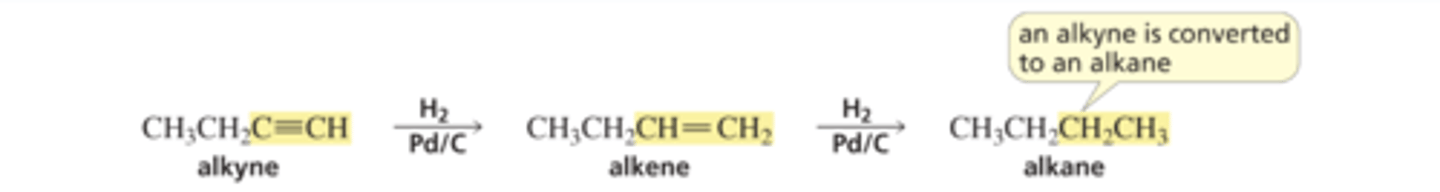
Forming a cis Alkene from an Alkyne
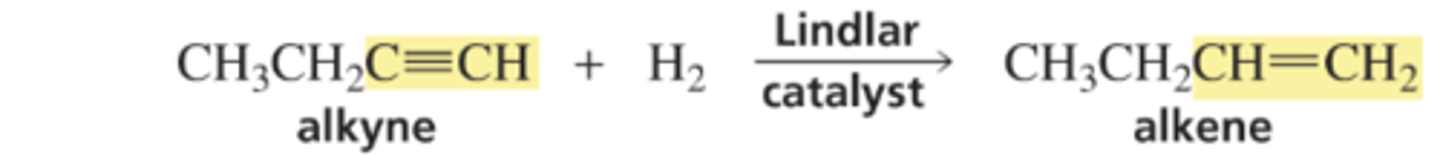
Mechanism for the Conversion of an Alkyne to a Trans Alkene
■ The single electron from the s orbital of sodium is transferred to an sp carbon of the alkyne. This forms a radical anion—a species with a negative charge and an unpaired electron. Notice that the movement of a single electron is represented by an arrowhead with a single barb. (Recall that sodium has a strong tendency to lose the single electron in its outer-shell s orbital; Section 1.3.)
■ The radical anion is such a strong base that it can remove a proton from ammonia. This results in the formation of a vinylic radical—the unpaired electron is on a vinylic carbon.
■ Another single-electron transfer from sodium to the vinylic radical forms a vinylic anion.
■ The vinylic anion is also a strong base; it removes a proton from another molecule of ammonia, forming the trans alkene.
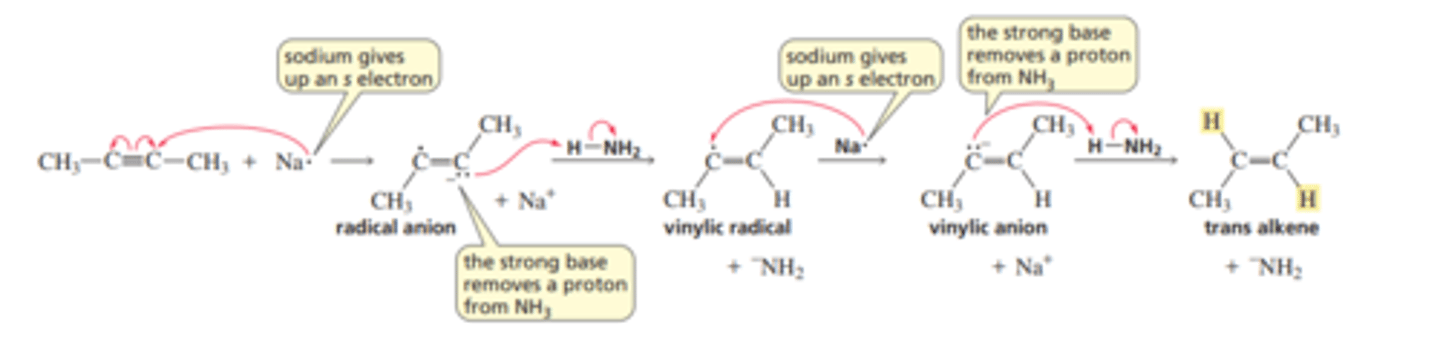
Acetylide ions
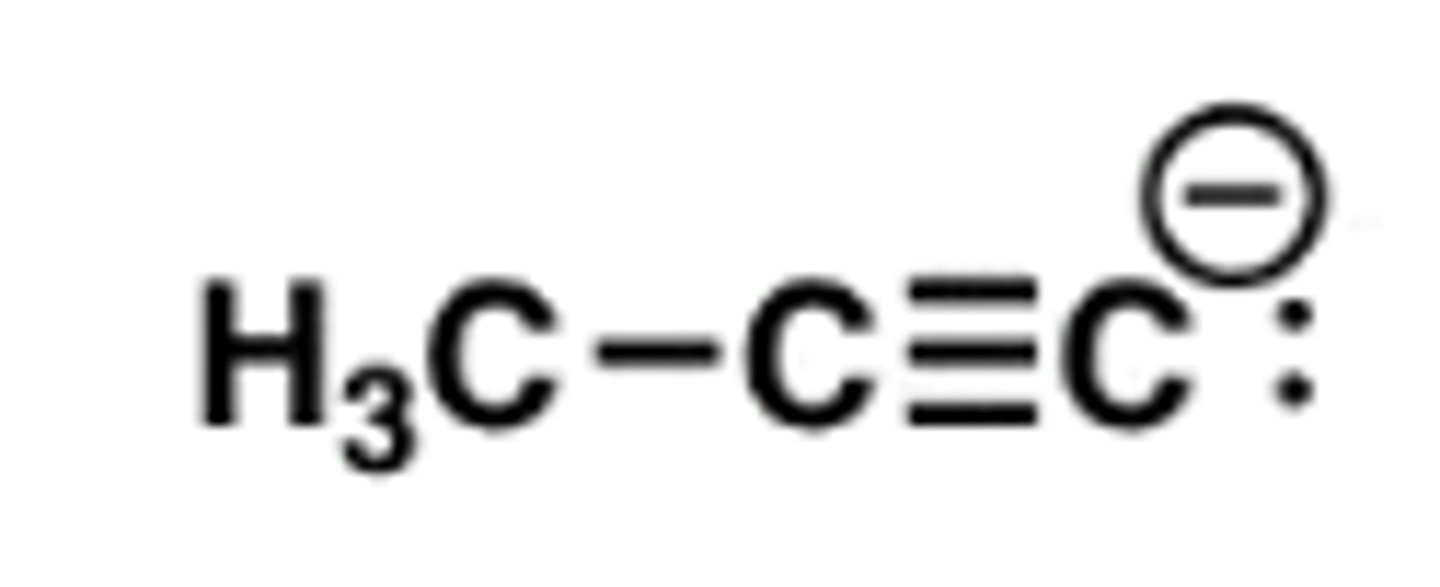
Synthesis Using Acetylide Ions
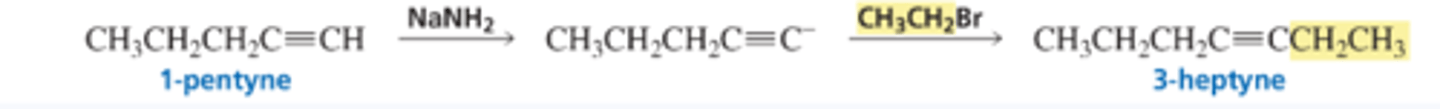
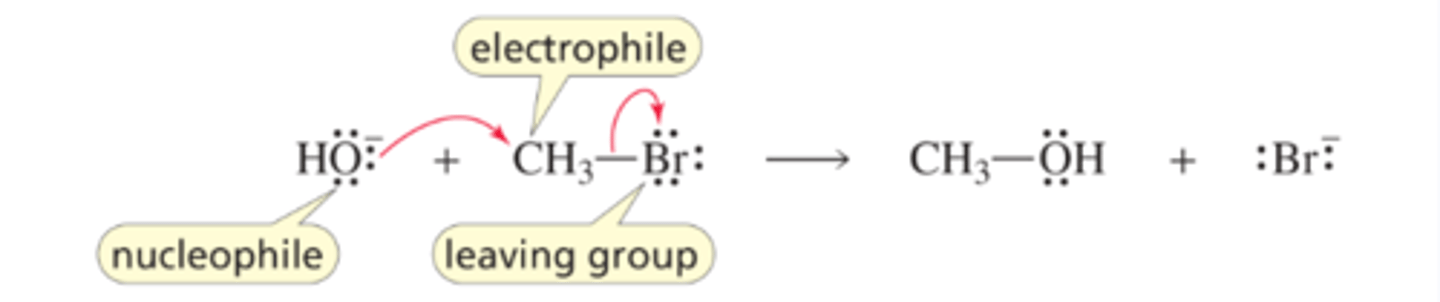
Mechanism for the SN2 Reaction of an Alkyl Halide
■ The nucleophile attacks the back side of the carbon (the electrophile) that bears the leaving group and displaces it.
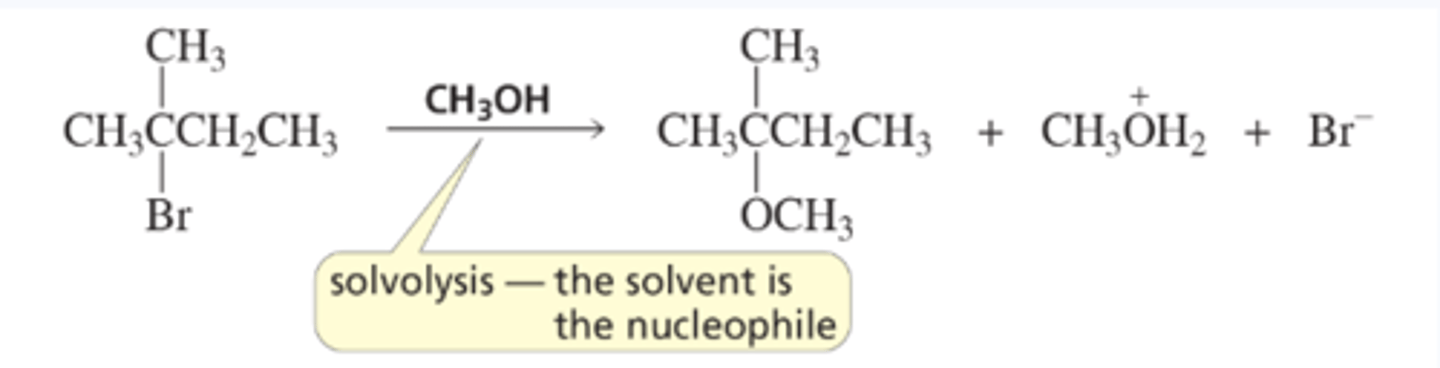
Solvolysis - the solvent is the nucleophile
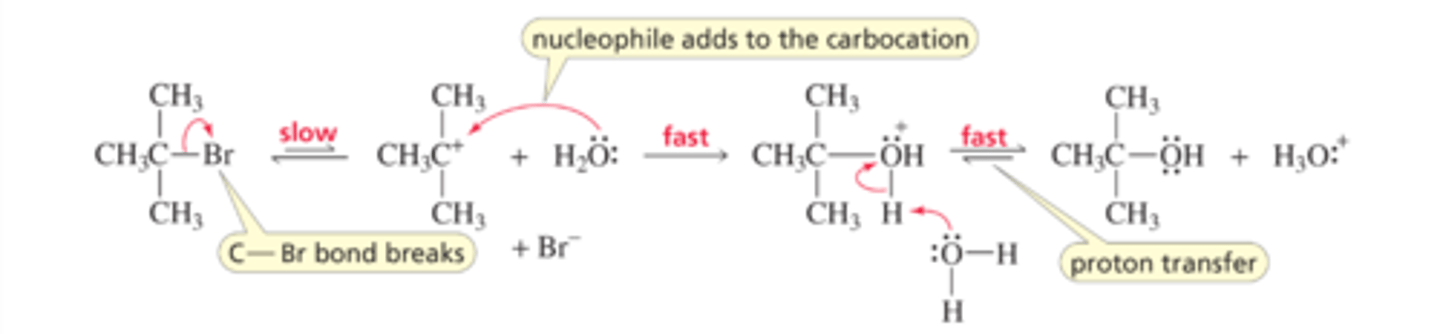
Mechanism for the SN1 Reaction of an Alkyl Halide
■ In the first step, the carbon-halogen bond breaks and the previously shared pair of electrons leaves with the halogen. As a result, a carbocation intermediate is formed.
■ In the second step, the nucleophile reacts rapidly with the carbocation (an electrophile) to form a protonated alcohol.
■ Whether the alcohol product exists in its protonated (acidic) form or neutral (basic) form depends on the pH of the solution. At pH = 7, the alcohol exists only in its neutral form (Section 2.10).
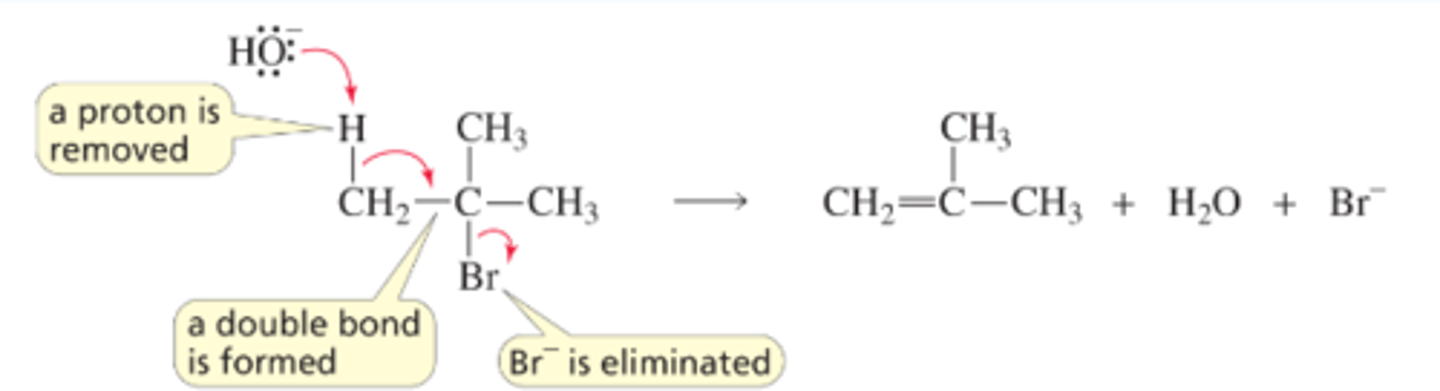
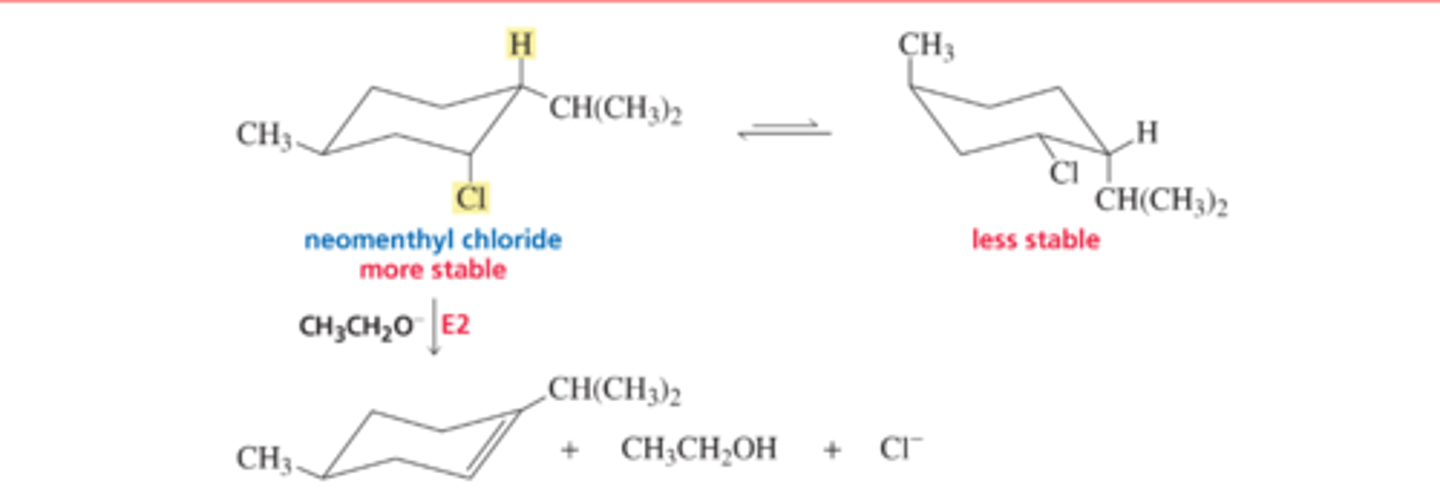
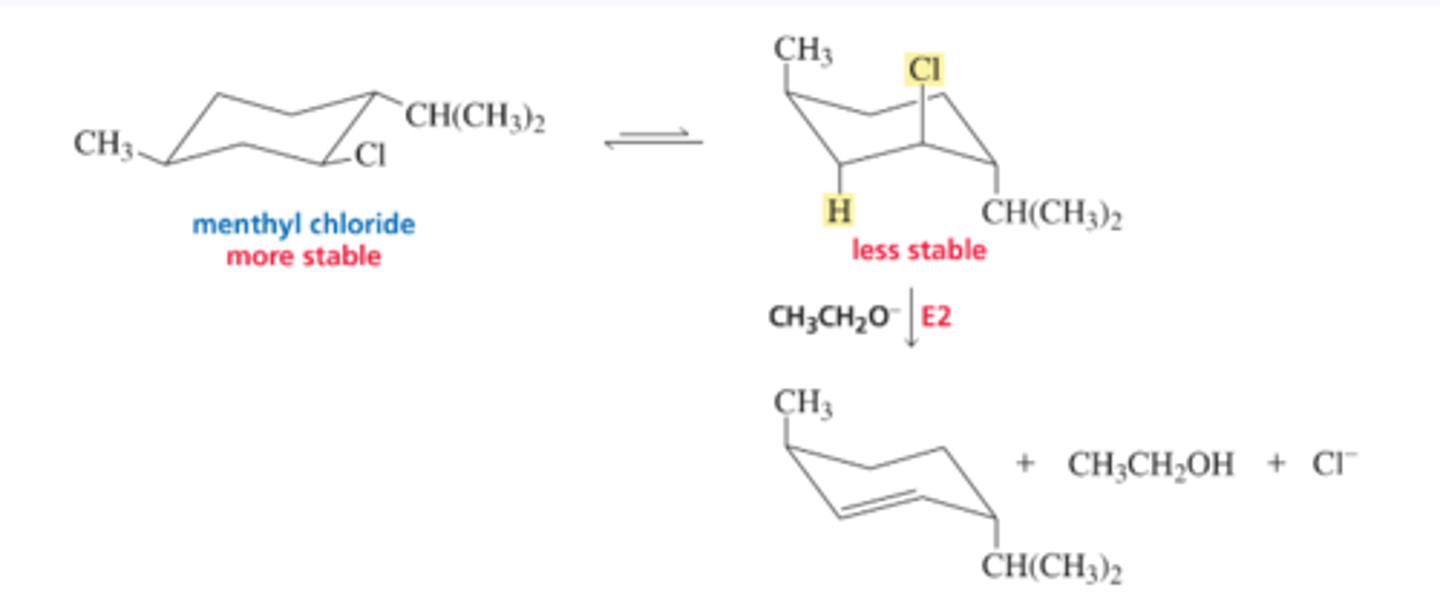
Mechanism for the E2 Reaction of an Alkyl Halide
■ The base removes a proton from a carbon that is adjacent to the carbon bonded to the halogen. As the proton is removed, the electrons that it shared with carbon move toward the carbon that is bonded to the halogen. As these electrons move toward the carbon, the halogen leaves (because carbon can form no more than four bonds), taking its bonding electrons with it
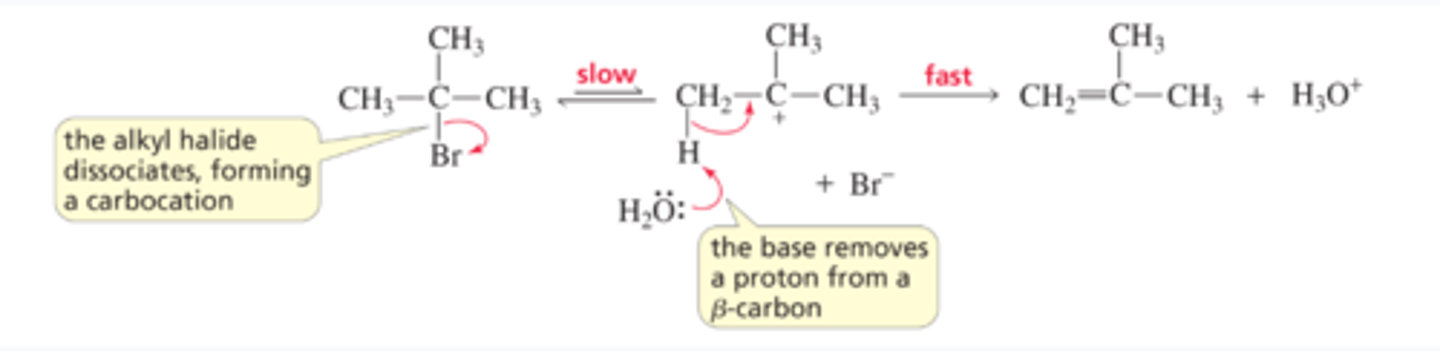
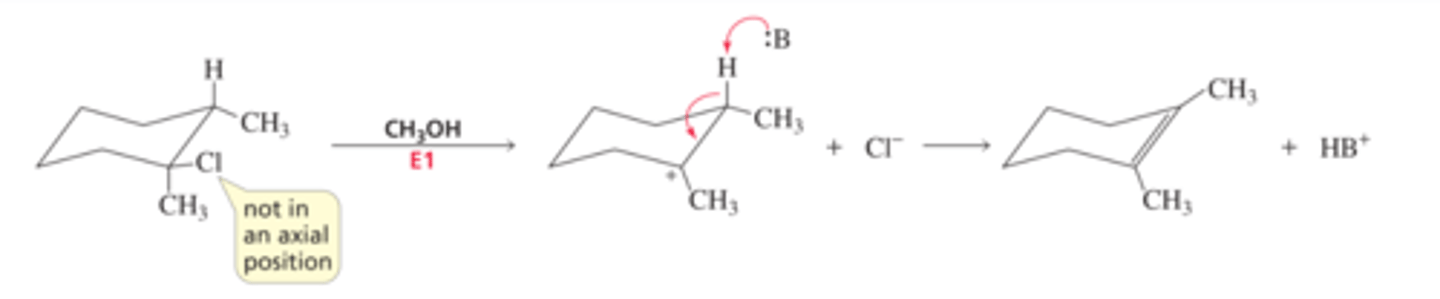
Mechanism for the E1 Reaction of an Alkyl Halide
■ The alkyl halide dissociates, forming a carbocation.
■ The base forms the elimination product by removing a proton from a b-carbon
E2 Reactions of Substituted Cyclohexanes: More stable conformer
E2 Reactions of Substituted Cyclohexane: Less stable conformer
E1 Reactions of Substituted Cyclohexanes
Williamson Ether Synthesis
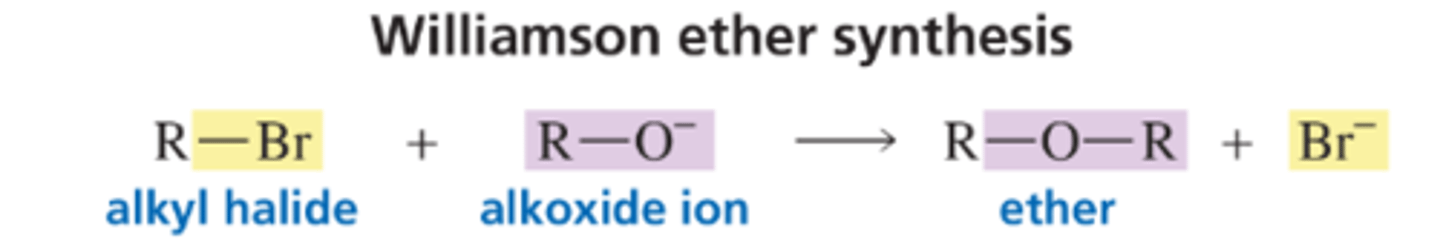
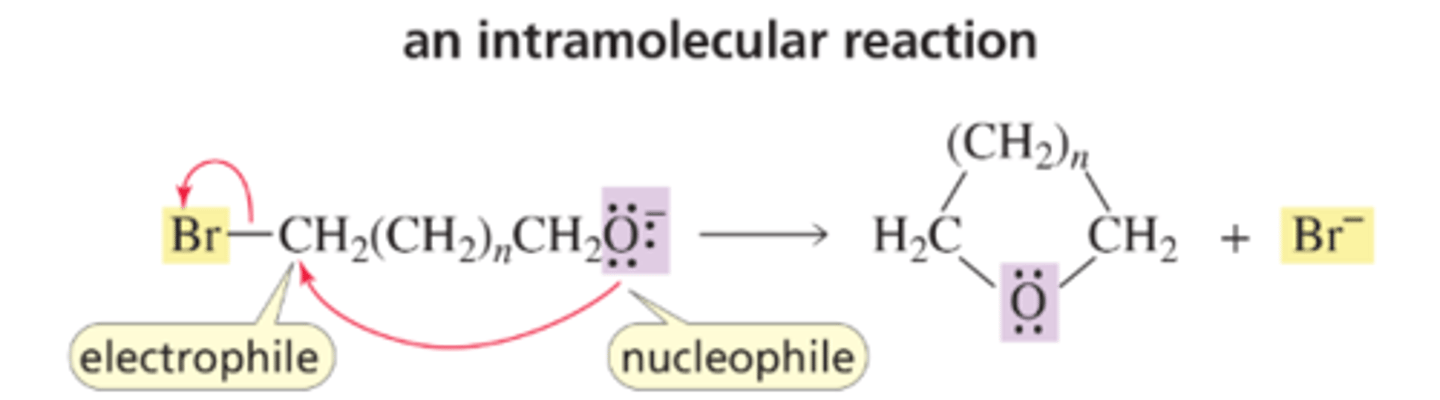
Intramolecular Reaction
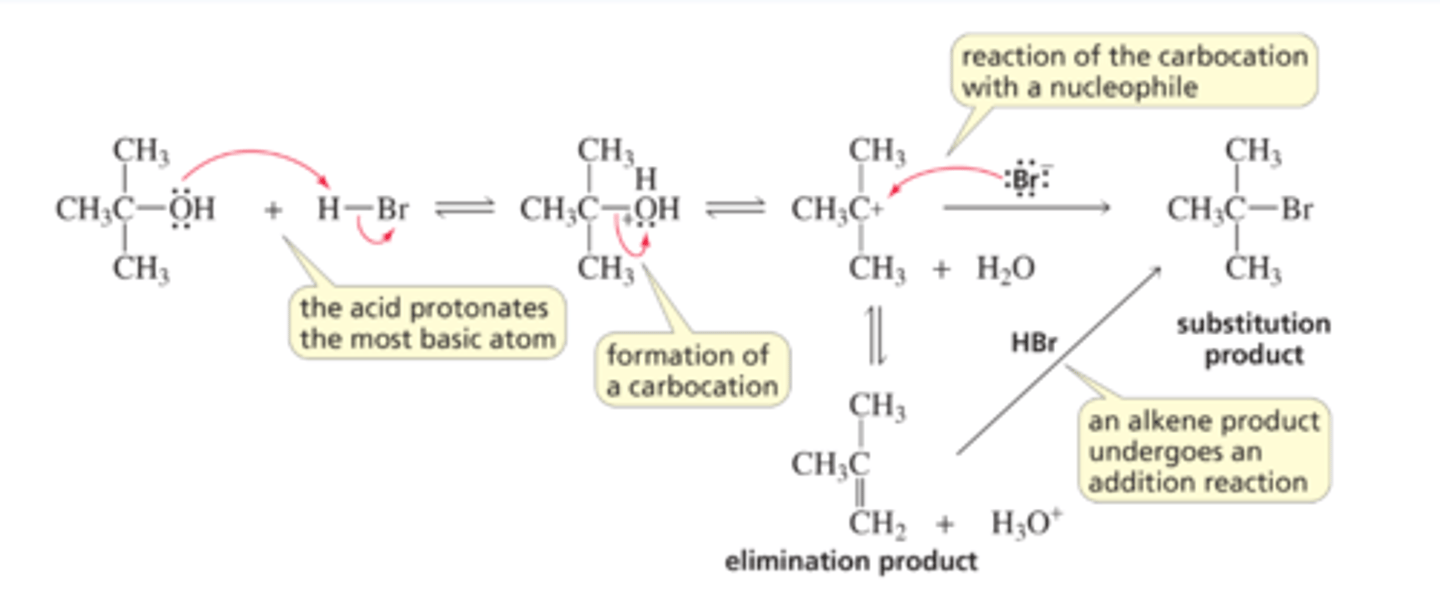
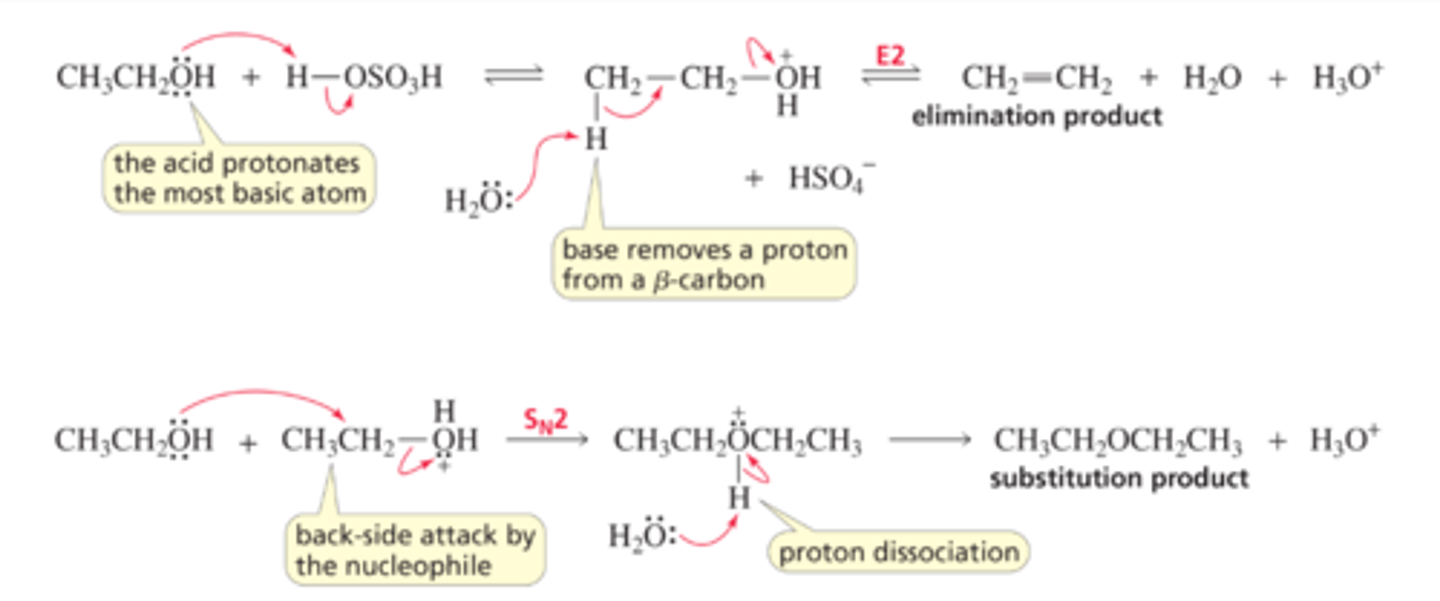
Mechanism for the SN1 Reaction of an Alcohol
■ An acid always reacts with an organic molecule in the same way: it protonates the most basic atom in the molecule.
■ Weakly basic water is the leaving group that is expelled, forming a carbocation.
■ The carbocation, like the carbocation formed when an alkyl halide dissociates in an SN1 reaction, has two possible fates: it can combine with a nucleophile and form a substitution product, or it can lose a proton and form an elimination product (Section 9.12).
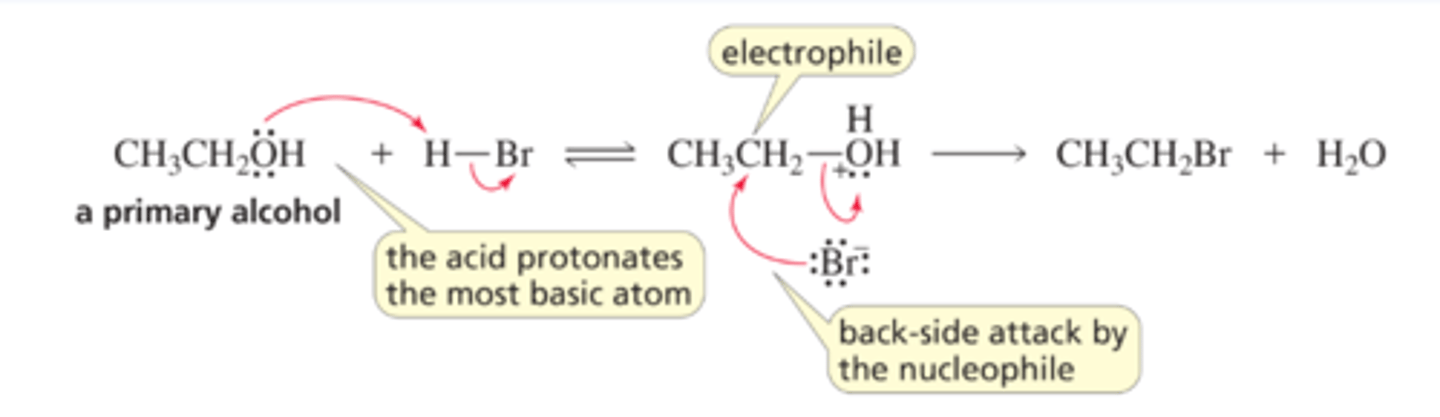
Mechanism for the SN2 Reaction of an Alcohol
■ The acid protonates the most basic atom in the reactant.
■ The nucleophile attacks the back side of the carbon and displaces the leaving group.
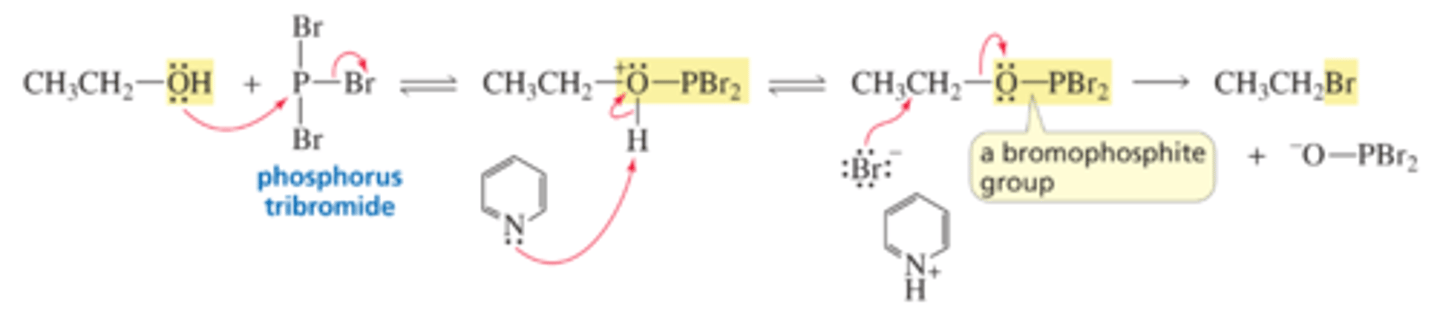
Mechanism for the Conversion of an Alcohol to an Alkyl Bromide (or Alkyl Chloride) Using PBr3 (or PCl3)
■ The first step is an SN2 reaction on phosphorus.
■ Pyridine is generally used as a solvent in these reactions because it is a poor nucleophile. However, it is sufficiently basic to remove a proton from the intermediate, which prevents the intermediate from reverting to starting materials.
■ The bromophosphite group is a weaker base than a halide ion, so it is easily displaced by a halide ion.
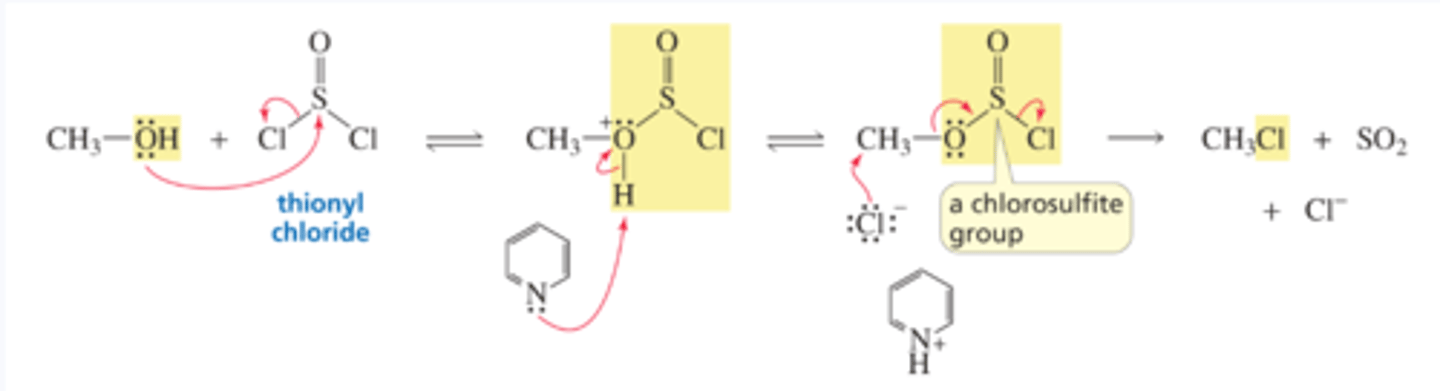
Mechanism for the Conversion of an Alcohol to an Alkyl Chloride Using SOCl2
■ The first step is an SN2 reaction on sulfur.
■ Pyridine removes a proton from the intermediate, which prevents the intermediate from reverting to starting materials.
■ The chlorosulfite group is a weaker base than a chloride ion, so it is easily displaced by a chloride ion
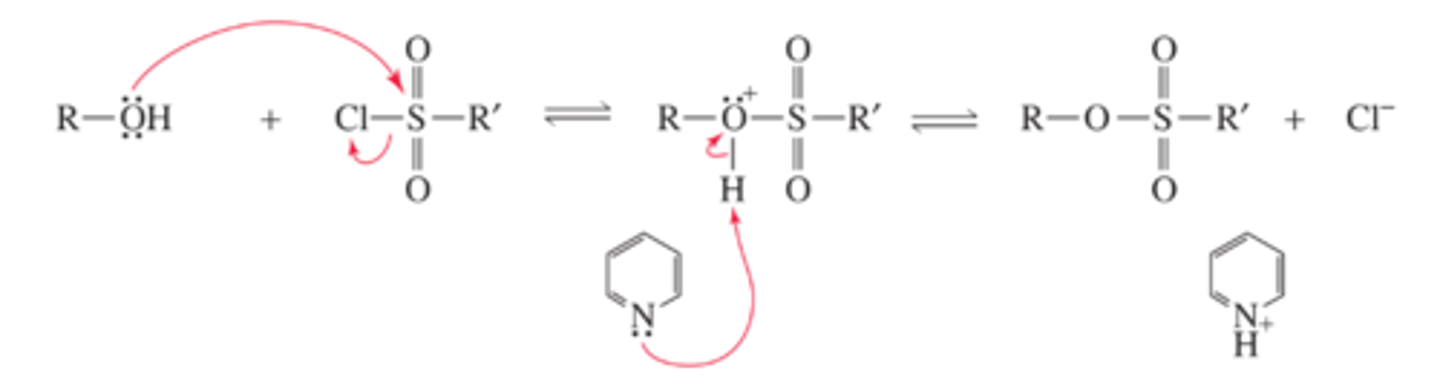
Mechanism for the Conversion of an Alcohol to a Sulfonate Ester
■ The first step is an SN2 reaction on sulfur.
■ Pyridine removes a proton from the intermediate, which prevents the intermediate from reverting to starting materials.
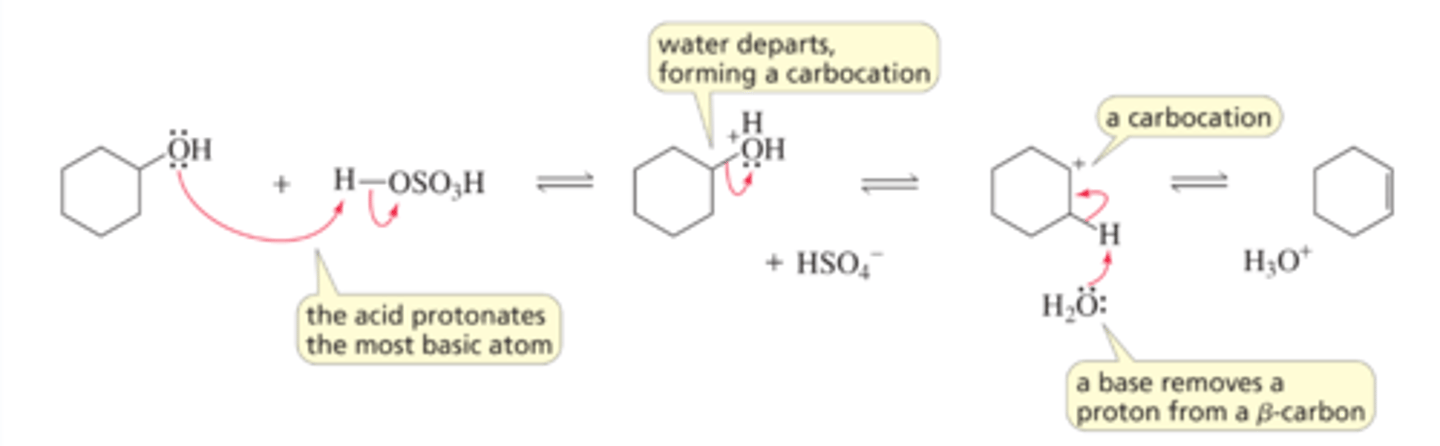
Mechanism for the E1 Dehydration of an Alcohol
■ The acid protonates the most basic atom in the reactant. As we saw earlier, protonation converts the very poor leaving group (HO-) into a good leaving group (H2O).
■ Water departs, leaving behind a carbocation.
■ A base in the reaction mixture (water is the base that is present in the highest concentration) removes a proton from a b-carbon (a carbon adjacent to the positively charged carbon), forming an alkene and regenerating the acid catalyst. Notice that the dehydration reaction is an E1 reaction of a protonated alcohol.
Mechanism for the E2 Dehydration of a Primary Alcohol and for the Competing SN2 Reaction
The reaction also forms an ether in a competing SN2 reaction, because primary alcohols are the ones most likely to form substitution products under SN2/E2 conditions. However, the elimination reaction is favored because of the high temperature required for the dehydration reaction (Section 9.12). Because the dehydration of a primary alcohol is an E2 reaction, we expect 1-butene to be the product of the E2 dehydration of 1-butanol. The product, however, is actually 2-butene. 1-Butene is the initial product but after it forms, a proton from the acidic solution adds to the double bond (adding to the sp2 carbon bonded to the most hydrogens in accordance with the rule that governs electrophilic addition reactions), thereby forming a carbocation (Section 6.4). Loss of a proton from the b-carbon bonded to the fewest hydrogens (Zaitsev's rule) forms 2-butene (Section 9.8).
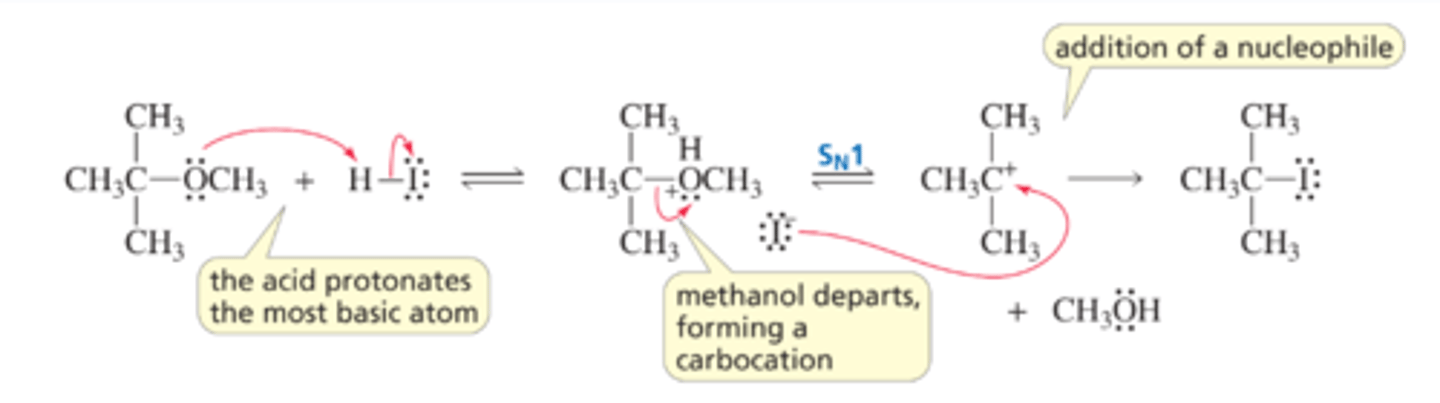
Mechanism for Ether Cleavage: An SN1 Reaction
■ The acid protonates the oxygen, thereby converting the very basic RO- leaving group into the less basic ROH leaving group.
■ The leaving group departs, forming a carbocation.
■ The halide ion combines with the carbocation.
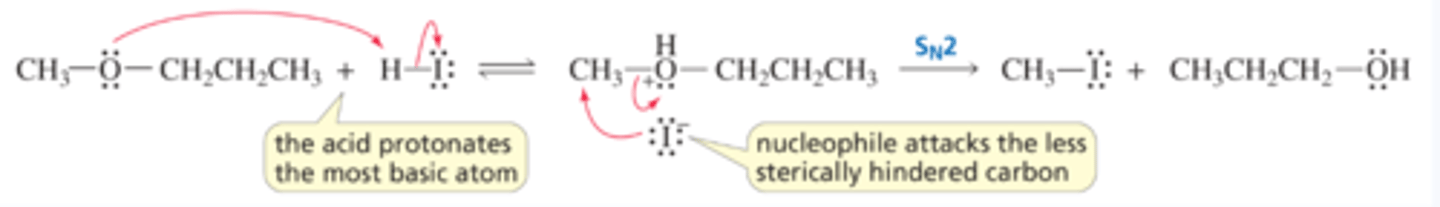
Mechanism for Ether Cleavage: An SN2 Reaction
■ Protonation converts the very basic RO- leaving group into the less basic ROH leaving group.
■ The halide ion preferentially attacks the less sterically hindered of the two alkyl groups.
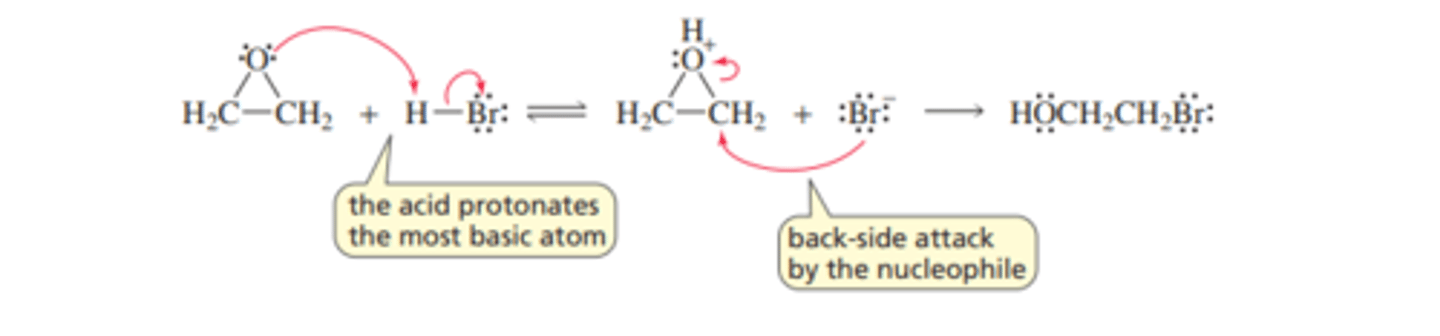
Mechanism for Nucleophilic Substitution: Acidic Conditions
■ The acid protonates the oxygen of the epoxide.
■ The protonated epoxide undergoes back-side attack by the halide ion.
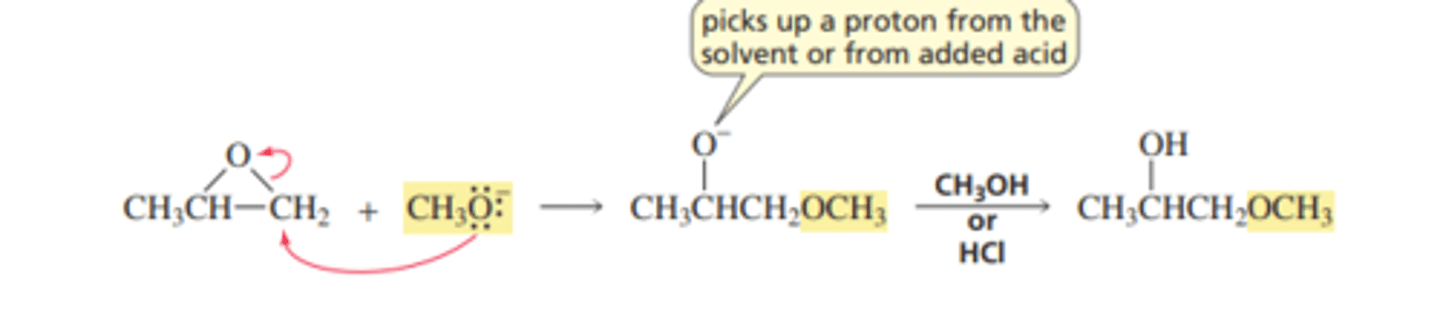
Mechanism for Nucleophilic Substitution: Neutral or Basic Conditions
■ The C¬O bond does not begin to break until the carbon is attacked by the nucleophile. The nucleophile is more likely to attack the less substituted carbon because it is less sterically hindered.
■ The alkoxide ion picks up a proton from the solvent or from an acid added after the reaction is over
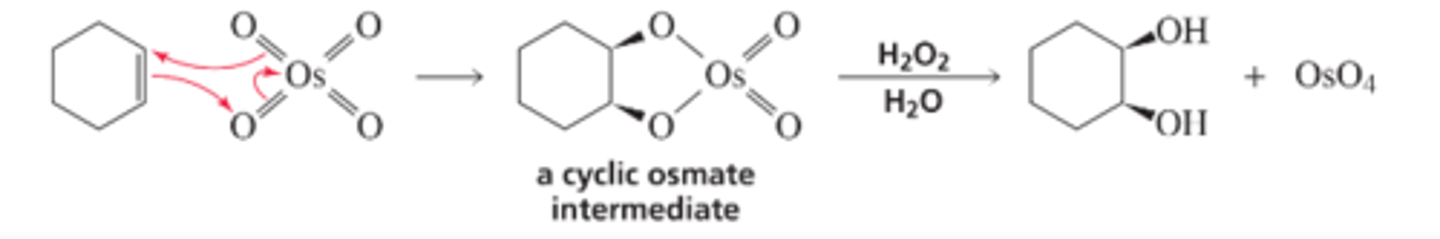
Mechanism for Cis-Glycol Formation
■ Osmium tetroxide forms a cyclic intermediate when it reacts with an alkene.
■ The intermediate is hydrolyzed with aqueous hydrogen peroxide. Hydrogen peroxide re-oxidizes the osmium reagent back to osmium tetroxide. (Because osmium tetroxide
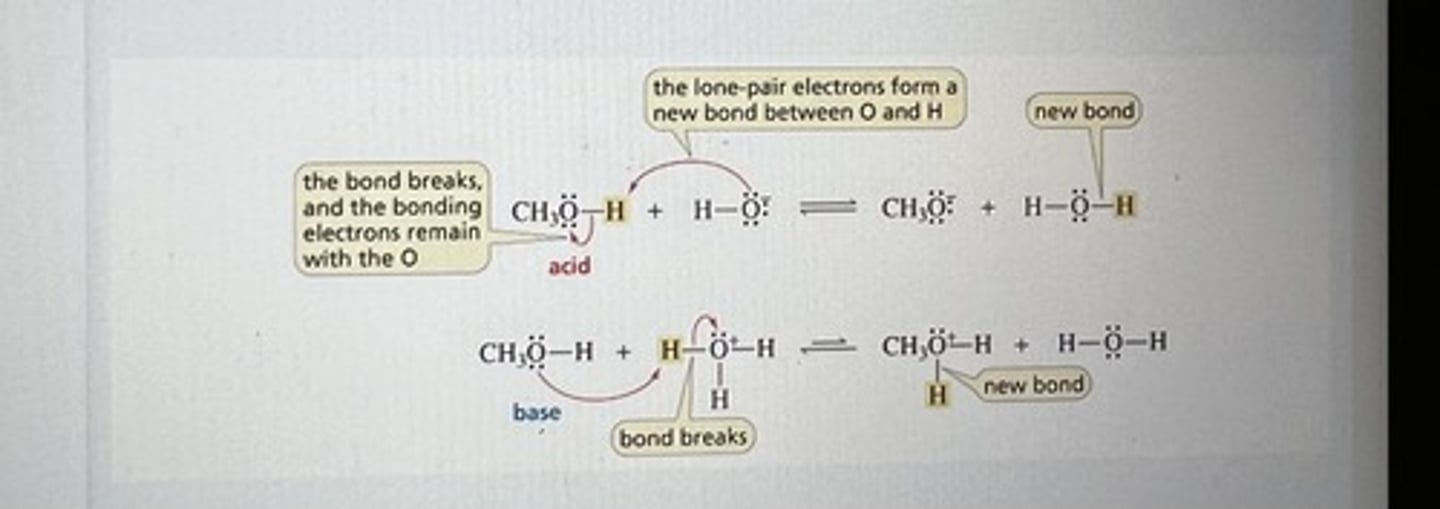
Acid-Base Reaction