8 vet medicines
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
What are Veterinary Medicines? (4)
Products/substances used to treat or prevent disease in animals
Restore, correct, or modify physiological functions
by Exert pharmacological, immunological, or metabolic actions
Apply to animals other than humans
what is the Veterinary Medicines Directorate (VMD)?
Executive agency of DEFRA (Department for Environment, Food and Rural Affairs)
Focuses on public health protection and animal welfare standards
What are the Key Roles and Responsibilities of the VMD?
Enforce Veterinary Medicines Regulations 2013 (VMR)
Monitor and manage adverse drug events (ADEs) in veterinary medicines
License and authorize veterinary medicines (Marketing Authorization)
Test veterinary medicines in animals/animal products
Authorize companies to sell veterinary medicines
Control production and distribution of veterinary medicines
Advise government ministers on veterinary medicines policy
Inspect premises in the veterinary medicine supply chain
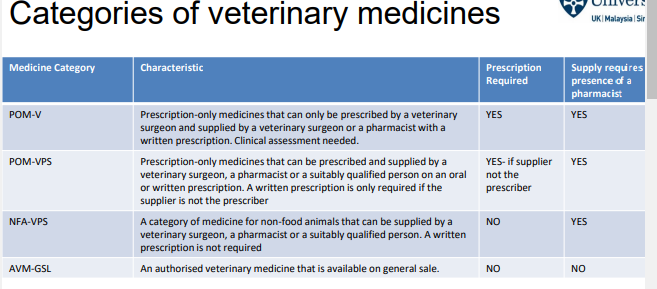
What is POM-V (Prescription-only Medicine - Veterinary)? (4_)
Can only be prescribed by a veterinary surgeon
Supplied by a veterinary surgeon or a pharmacist with a written prescription
Requires clinical assessment
Written prescription is mandatory
What is POM-VPS (Prescription-only Medicine - Veterinary, Prescription-only Supply)?
Can be prescribed by a veterinary surgeon, pharmacist, or suitably qualified person
Can be supplied by the same or different person,
Written prescription required only if supplier is not the prescriber
what is NFA-VPS (Non-Food Animal - Veterinary, Prescription-only Supply)?
Medicines for non-food animals
Can be supplied by a veterinary surgeon, pharmacist, or suitably qualified person
No written prescription required
What is AVM-GSL (Authorised Veterinary Medicine - General Sale)?
Available on general sale
No written prescription required
Can be bought without restrictions
What is an Unauthorised Veterinary Medicine?
An unlicensed medicine without marketing authorisation
Not eligible for exemption through the SAES (Specialist Use in Exemption Scheme)
Can only be prescribed by a veterinary surgeon under the Cascade
Includes human medicines used for animals
Requires a prescription for supply
What are the Key Elements of a Veterinary Prescription?
Prescriber Info: Name, address, phone, qualifications, and signature (RCVS number for Sch 2 & 3 CDs).
Owner Info: Name and address.
Animal Info: Identification, species, and address (if different from owner).
Date: Prescriptions valid for 6 months or less (28 days for Schedule 2, 3, and 4 CDs).
Medicine Details: Name, quantity, dose, and administration instructions (no “as directed”).
Warnings: Any relevant warnings, including withdrawal period for food use.
Cascade: If prescribed under the Cascade, note it.
Repeatable: Indicate the number of repeats (if applicable)
What is Required for Schedule 2 or 3 Controlled Drugs (CDs) Prescriptions?
A declaration must state: "The item has been prescribed for an animal or herd under the care of the veterinarian."
Usual controlled drugs prescription requirements must also apply.
Are Standardised Forms Required for Veterinary Prescriptions?
No, standardised forms are not required for veterinary prescriptions.
How Long Should Veterinary Prescriptions be Retained?
should they be sent to nhs agency
Veterinary prescriptions should be kept for 5 years.
They should not be submitted to the relevant NHS agency.
What is the Recommended Duration for Controlled Drugs (CDs) Prescriptions?
Good practice to prescribe a maximum of 30 days of treatment for CDs.
Same as human CD prescription guidelines, unless long-term medication is required.
What are the Legal Requirements for Pharmacists Supplying NFA-VPS or Prescribing POM-VPS Medicines? 4
Advise on safe usage of the product
Highlight applicable warnings and contraindications from the packaging label
Ensure the recipient intends to use the medicine correctly and is competent
Prescribe or supply the minimum quantity needed for treatment
Are There Additional Controls for Certain Veterinary Medicines?
Yes, food-producing animals, horses, and specific veterinary medicines are subject to extra controls and considerations.
What is the Cascade in Veterinary Medicine?
Under VMR, only authorised veterinary medicines can be prescribed/administered to animals.
If no authorised product exists, a veterinary surgeon can prescribe a medicine ‘under the cascade’,
What is the Rule for Selling Human Medicines for Animal Use?
It is unlawful to sell or supply human medicines (including GSL or P medicines) for animal use, unless prescribed under the cascade.
What Are the Requirements for Supplying POM-V, POM-VPS, and NFA-VPS Medicines?
Physical presence of a pharmacist is required for the supply of these medicines, unless:
The transaction has been authorised in advance by a pharmacist.
The person handing out the prescription is judged to be competent.
Is There a Legal Requirement to Label Authorised Veterinary Medicines in Their Packaging?
No legal requirement to label authorised VMPs in their authorised packaging.
However, detailed labelling and packaging requirements exist for authorised veterinary medicines.
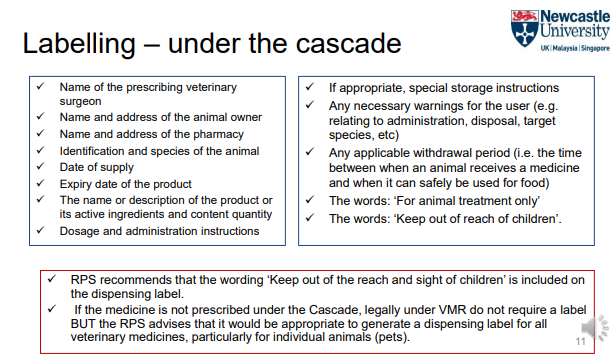
What Does the RCVS Require for Labels on Dispensed Veterinary Medicines?
Care information on packaging must not be obscured.
Labels must include:
Name and address of the animal owner.
Name and address of the veterinary practice.
Date of supply.
Name, strength, and quantity of the product.
Dosage and directions for use.
'For animal treatment only'.
'For external use only' (for topical preparations).
What Are the Labelling Requirements When Supplying Veterinary Medicines by Retail?
If supplied by retail, information on the packaging must be clearly visible.
What are the Record-Keeping Requirements for POM-V and POM-VPS Medicines?
Keep records of receipts and supply of POM-V and POM-VPS products.
Records must be durable, permanent, and available for inspection.
Records can be kept electronically.
What Information Must Be Included in the Records?
Name of the medicine
Date of receipt or supply
Batch number
Quantity
Name and address of the supplier or recipient
If there is a prescription, include the prescriber’s name and address, and keep a copy of the prescription.
How Long Must Records Be Kept and What Additional Requirements Are There?
Records must be kept for at least 5 years.
Pharmacies must undertake an annual audit of POM-V and POM-VPS medicines.
What is Wholesale Supply in Veterinary Medicines?
Wholesale supply refers to all supplies of veterinary medicines that are not retail.
Who Can Supply Veterinary Medicines to Retailers?
Only the manufacturer of a veterinary medicine or a holder of a Wholesale Dealer’s Authorisation (WDA) can routinely supply authorised retailers.
Can Authorised Retailers Supply to Other Retailers Without a WDA?
Yes, authorised retailers may supply medicines to other authorised retailers to relieve a temporary supply shortage without a WDA, but only to prevent animal welfare issues.
This exemption does not apply to wholesale supply, which still requires a WDA.
What Is Required to Supply Human Medicines for Veterinary Use?
To supply human medicines for veterinary use under the cascade, you must hold a WDA(H) issued by the Medicines and Healthcare products Regulatory Agency (MHRA).
What are Some Offences Related to Veterinary Medicines?
