I Structure of matter - flashcards
1/71
Earn XP
Description and Tags
all-in-one flashcards for the first section <3
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
Atomic weight (average atomic mass)
= Sum of isotope masses multiplied by their abundance / abundance of all isotopes (usually 100)
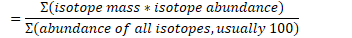
Molecular or formula weight
A compound’s or molecule’s sum of the atomic weights of all the individual atoms in the molecule
Molar mass
Element’s atomic mass in grams per moles
Amount of substance
Measures in moles. One mole is defined as the Avogadro’s number’s amount of particles.
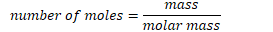
Molar concentration (molarity)
number of moles of solute per liter of solution (mol/L)
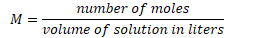
Expressed: [Na+] "the molar concentration of sodium ions"
Mass percent concentration (weight percent)
ratio of the mass of the solute to the total mass of the solution in %
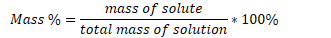
The ideal gas law equation
pV=nRT
R = ideal gas constant
What are ionic bonds?
Atomic bonding where atoms transfer electrons, creating charges, which results in an electrostatic attraction that holds the ions together and forms the ionic bond.
between metals and nonmetals or between polyatomic ions
What is a cation?
Positive ion, lost electrons
What is an anion?
Negative ion, gained electrons
What is needed for an ionic bond to form?
The electronegativity difference between elements has to be over 1.7.
What are 5 typical properties of ionic bonds?
High melting and boiling points
Hardness and brittleness
Electrical conductivity 0 when solid
Soluable in water (polar)
Crystal shape (ionic crystal)
What is ionization energy? (ionization potential)
The amount of energy needed to remove an electron from an isolated atom or molecule
What is electron affinity EA?
A measure of the change of energy when an electron is added to a natural atom in the gas phase to form an anion
basically the opposite to ionization energy
high ea (negative ea) indicates a strong attraction for the extra electron and so on
ea describes the atom’s system’s energy, so negative means that it’ll release energy to take..
What significance does electron affinity have?
Chemical Reactivity
Elements with high (more negative) electron affinities tend to be more reactive nonmetals
Predicting Ionic Bonds
Electron affinity helps predict the likelihood of an atom forming an anion
How are covalent bonds formed?
When atoms share electron pairs.
goal is to attain the electron configuration of a noble gas ( a stable molecule )
What is a coordinate bond ( dative covalent bond )?
A covalent bond where both electrons in the shared bond come from the same atom
two atoms of the same element bond together
What is electronegativity?
A measure of an atoms ability to attract and hold onto electrons in a covalent bond
What is polarity or nonpolarity in covalent bonds?
Polar = “unequal sharing” of the electrons, where there are regions of distinct partial positive or partial negative charges.
electronegativity difference is 0.5 to 1.7
Nonpolar = equal sharing of the electrons, where the molecule is neutral in charge.
electronegativity difference of 0 to 0.4
the molecular geometry could cancel out charges, making it a nonpolar molecule.
What are the typical properties of covalent bonds?
Low melting and boiling points, due to the intermolecular forces being generally weaker than the bonds themselves
Polar covalent compounds are soluble in polar solvents (water)
Nonpolar covalent compounds are soluble in nonpolar solvents (oil)
Not conductive (exception of graphite in electrical conductivity)
Molecular structures; each molecule is their own "unit" instead of a continuous lattice seen in ionic or metallic compounds.
What are intramolecular forces?
Forces that hold together atoms within a molecule
very strong compared to intermolecular forces
responsible for the chemical properties of a substance
covalent, ionic, metallic
What are intermolecular forces?
Forces of attraction or repulsion between molecules
determines the physical properties of a substance (melting and boiling point, vapor pressure, solubility)
Van der Waals forces, hydrogen bonds
What are van der Waals forces and name the three types.
Bonds resulting from weak intermolecular forces between molecules or atoms
significant in determining the physical properties of substances (boiling point, melting point, solubility)
London dispersion forces
dipole-dipole interactions
dipole-induced dipole interactions
What are London dispersion forces, how do they come to be and what affects the strength?
Weak attractions due to temporary fluctuations in the electron distribution within atoms or molecules, regardless of the polarity.
Strength increases with the size and shape of the molecule, because of the larger area where the temporary dipoles can form.
What are dipole-dipole interactions?
Interactions between polar molecules due to electrostatic attractions
generally stronger than London dispersion forces
What are dipole-induced dipole interactions?
A polar molecule can distort the electron cloud of a nearby nonpolar molecule, inducing a temporary dipole
generally stronger than London dispersion forces, but weaker than dipole-dipole interactions
Rank intermolecular forces from strongest to weakest
Hydrogen bond
Dipole-dipole interactions
dipole-induces dipole interactions
London dispersion forces
What are hydrogen bonds?
strong type of dipole-dipole interaction
A hydrogen atom is covalently bonded to a highly electronegative atom (most commonly nitrogen (N), oxygen (O) or fluorine (F)) which then interacts with a lone pair of electrons on another electronegative atom in a nearby molecule
Lone pair of electrons = pairs of valance electrons not shared with another atom and are not thus involved in bonding
Provide a region of negative charge
What are subatomic particles?
The particles that make up atoms; protons, neutrons, electrons
What is the charge, weight and effect of an electron (in an atom)
negative charge of -1 or 1-
mass of about 0.0005u
determines the chemical properties of an atom
What is the charge, mass and meaning of a proton (-s in an atom)
positive charge of 1+ or +1
mass of 1u
# of protons determines the element’s identity ( Z )
What is the charge, mass and contribution that a neutron has (to an atom)
no charge (0)
similar mass to protons of 1u (slightly larger)
contribute to the mass of the atom and help stabilize the nucleus
What is the atomic number of an atom?
Amount of protons in the nucleus, which is unique to each element
How is mass number A calculated?
A = # of protons + # of neutrons
What’s the average atomic mass (atomic weight) of an element
Element’s weighted average mass of the atoms in a naturally occurring sample of the element
What is an isotope?
Atoms of the same element, but with different mass numbers.
What are the four inner layers of electrons’ letters?
K (n=1), L (n=2), M (n=3), N (n=4)
How is the maximum amount of electrons in an electron shell calculated?
2n²
What’s the difference with the Bohr model and the Lewis diagram?
The Bohr model includes all the shells of the electrons, where the Lewis diagram only includes the valance shell
Name the four electron subshells. How do they work and how many electrons can be in each subshell?
The four subshells are s,p,d,f.
Each electron shell has different subshell combinations:
K = s
L = s, p
M = s, p, d
N = s, p, d, f
Within each subshell, there is an amount of orbitals:
s = 1
p = 3
d = 5
f = 7
and each orbital can hold up to 2 electrons.
What significance do valance electrons hold?
They determine most of an atom’s chemical behaviors
why elements in the main groups have similar behaviors and similar ionic compounds (helium is an exception)
What is the periodic law on atomic radius?
Atomic radius decreases across a period and up a group
explanation:
Across a period (left to right): Atomic radius decreases because the number of protons increases, pulling the electrons closer to the nucleus due to a stronger electrostatic attraction.
Up a group (bottom to top): Atomic radius also decreases because the number of electron shells reduces as you move up the group.
What is the periodic law on ionization energy?
Ionization energy increases across a period and up a group
explanation:
Across a period: Ionization energy increases because atoms have more protons, making it harder to remove an electron due to stronger nuclear attraction.
Up a group: Ionization energy increases because atoms have fewer electron shells, so the outermost electrons are closer to the nucleus and more tightly held.
What is the periodic law on electronegativity?
Electronegativity increases across a period and up a group
explanation:
Across a period: Electronegativity increases as the number of protons grows, leading to a stronger pull on shared electrons in a bond.
Up a group: Electronegativity increases because atoms are smaller, and the nucleus exerts a stronger attraction on bonding electrons.
Isotope
Atoms of the same element but with different amounts of neutrons.
have similiar or even identical chemical behavior, but may have differing physical properties (density, radioactivity, etc)
isotopes can be stable or unstable
written using isotope notation
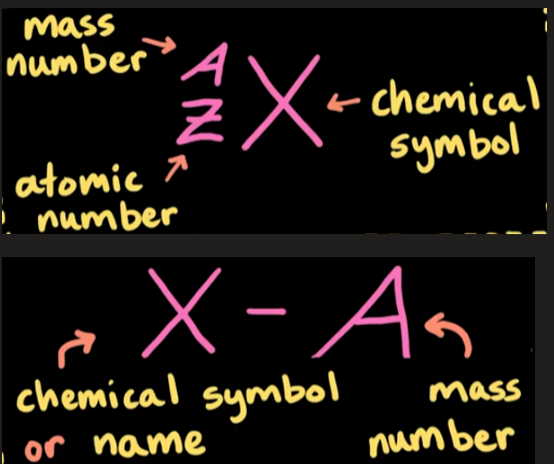
Allotrope
Different forms of the same element, where the atoms are arranged in distinct ways
differing physical and chemical properties (ie, conductivity)
carbon: diamond, graphite, graphene, fullerene
Molecule
2 or more atoms that are held together by (most commonly) covalent bonds
sizes range from diatomic molecules to complex macromolecules
Compound
Substance composed of two or more different elements that are chemically bonded together
ionic, covalent or metallic bonds
have fixed ratios depending on the atoms
need chemical methods to break the bonds
Mixture
Combination of two or more substances, where each substance retains its own chemical properties
no chemical bonds between the substances, meaning that they can be separated by physical means
Homogeneous mixtures
Mixtures uniform in composition and appearance throughout
solution
alloy
gaseous mixtures
colloids
Solution
Homogeneous mixture where a solute is dissolved in a solvent
can be separated by physical processes like evaporation or filtration
Alloy
Homogeneous mixture of metals
Gaseous mixture
Homogeneous mixture of gas
distribute uniformly
most commonly separated by distillation or fractional distillation, chromatography and absorption
Colloids
Homogeneous or heterogeneous mixtures depending how well the other substance is distributed throughout the other one.
for example whipped cream has two distinct phases (gas, liquid) which makes it heterogeneous, but milk is also a colloid, but homogeneous since the fat that it contains is well uniformly distributed throughout
for homogeneous colloids, ways of separation include
ultrafiltration
centrifugation
electrophoresis (separated using an electric field)
for heterogeneous colloids, ways of separation include
filtration and sieving
settling and decantation
centrifugation
Suspensions
Heterogeneous mixture where solid particles are dispersed throughout a liquid or gas, undissolved
can be separated by means of:
filtration
decantation
centrifugation
evaporation
magnetism
Heterogeneous mixtures
Mixtures not uniform and as such, the individual components can be seen and distinguished from one another
suspensions
colloids
emulsions
mechanical mixtures
Emulsion
Heterogeneous mixture that is a subtype of colloids, where the dispersed phase and continuous phase are both liquids
may be so unstable that separates over time
can be separated by means of
centrifugation
decantation
heating breaks the emulsion
addition of emulsifying agent, destabilizing the emulsion
filtration in some cases
Mechanical mixtures
Heterogeneous mixtures where the components are physically distinct and can be easily separated by filtration
Distillation
Way of separating homogeneous mixtures by utilizing differences in the boiling points of the components
Evaporation
Way of separating homogeneous mixtures by utilizing difference in boiling points or the volatility (vaporization ability)
Fractional distillation
Way of separating homogeneous mixtures with close but not the same boiling points, may include multiple steps
Chromatography
Way of separating homogeneous or heterogeneous mixtures based on their different ways of moving from stationary to mobile phases (move at different rates through a tube)
Ultrafiltration
Way of separating homogeneous mixtures where semi-permeable membrane is used to separate particles based on size (water purification)
Centrifugation
Using centrifugal force to separate components of a homogeneous or heterogeneous mixture based on density (spinning at high speeds to separate)
Filtration, sieving
Way of separating heterogeneous mixtures by utilizing differences in particle size
Settling and decantation
Way of separating heterogeneous mixtures by the settling of particles over time based on density
Magnetic seperation
Used to separate heterogeneous mixtures’ components when one is magnetic
Crystalline solid
Solid substance with a regular repeating structure (ie, diamond, NaCl)
Amorphous solid
Solid substance with no organized pattern in its lattice (glass)
Changes of state where energy is absorbed
Melting
Vaporization
Sublimation
Changes of state where energy is released
Freezing
Condensation
Deposition