Chapter 12 Preparation of salts
0.0(0)
Card Sorting
1/11
Earn XP
Description and Tags
Last updated 1:54 PM on 12/8/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
1
New cards
Phosphoric acid formula
Phosphoric acid creates?
Phosphoric acid creates?
HPO3
Phosphates
Phosphates
2
New cards
ethanoic acid formula
and it creates?
and it creates?
CH3COOH
ethanoates
ethanoates
3
New cards
nitric acid formula
and it creates?
and it creates?
HNO3
nitrates
nitrates
4
New cards
procedure to make salt pure dry crystals (3)
React acid and base to make salt solution
heat solution in evaporating basin (concentrate the solutio)
cool slowly form crystals
filter the crystals and dry them.
(if want can wash/ evaporate a few times)
heat solution in evaporating basin (concentrate the solutio)
cool slowly form crystals
filter the crystals and dry them.
(if want can wash/ evaporate a few times)
5
New cards
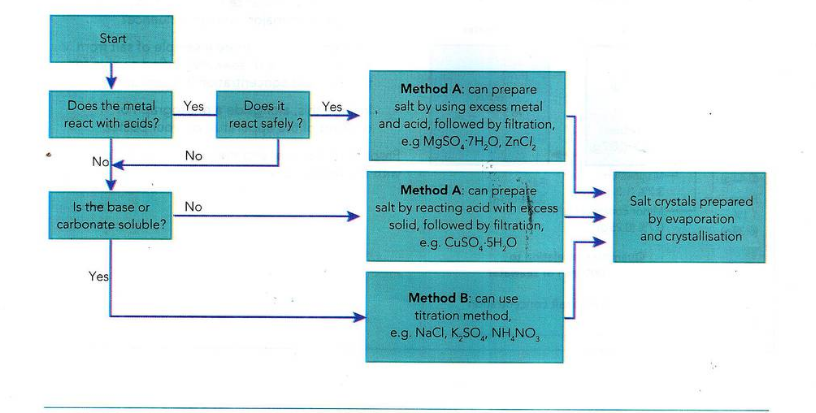
how to prepare soluble salt with acid alkaline (two methods) (2 x 3)
A
acid + solid (metal, base, or carbonate)
excess solid to make sure all acid is used.
NOTE bases/ carbonates are used instead of bare metal if the metal is too reactive.
B
acid + alkaline/ carbonate solution
used when bare metal is not reactive enough or when the base/carbonate is soluble
titration method
acid + solid (metal, base, or carbonate)
excess solid to make sure all acid is used.
NOTE bases/ carbonates are used instead of bare metal if the metal is too reactive.
B
acid + alkaline/ carbonate solution
used when bare metal is not reactive enough or when the base/carbonate is soluble
titration method
6
New cards
titration salt making procedure
do tritration to note how much of acid needed for base
then repeat but without using indicator
warm solution to concentrate it
cool slowly for crystal formation
filter and dry crystals
(if want can wash/ evaporate a few times)
then repeat but without using indicator
warm solution to concentrate it
cool slowly for crystal formation
filter and dry crystals
(if want can wash/ evaporate a few times)
7
New cards
chemically bonded water are called?
waters of crystallisation
8
New cards
what is filtrate?
fluid that which was filtered
9
New cards
residue
stuff that was filtered out
10
New cards
limiting reactant
substance that is not in excess
11
New cards
salt definition
product of acid + base
12
New cards
insoluble salt preparation
precipitation reaction (cannot use acid + solid as reaction will stop very fast, protective coating)
mix between two aqeous solutions, one containing the metal and the other the non-metal
for soluble solubtions of metal, can use NITRATES (all nitrate salts are soluble)
for soluble solutions of the non metal, can use SODIUM, POTASSIUM solutions (all soluble)
mix between two aqeous solutions, one containing the metal and the other the non-metal
for soluble solubtions of metal, can use NITRATES (all nitrate salts are soluble)
for soluble solutions of the non metal, can use SODIUM, POTASSIUM solutions (all soluble)