14 - Extraction and uses of Metals
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Location
Most metals are found in the Earth’s crust combined with other elements. These individual compounds are called minerals.
An ore is a sample of rock that contains enough of a mineral for it to be worthwhile to extract the metal.
Most metals are extracted from ores found in the Earth’s crust.
A few unreactive metals, such as gold, are found native which means they exist naturally as the un-combined element
Carbon extraction
For a metal below carbon in the reactivity series (carbon isn’t in the metal reactivity series but it would be right above zinc so think of it as “for a metal below zinc in the reactivity series“), the method of extraction is to heat it with carbon.
Iron is a good example for this. One of the main ores of iron contains a high percentage of iron(III) oxide. The iron can be extracted from this by heating it with carbon:
Fe₂O₃ (s) + 3C (s) → 2Fe(I) + 3CO (g)
Carbon is higher in the reactivity series than iron so it will take the oxygen away from the iron oxide. This is a redox reaction; the Fe₂O₃ is reduced to Fe in the reaction and the C is oxidised to CO. In this reaction, Carbon is the reducing agent, it reduces the iron(III) oxide.
Electrolysis
For a metal above carbon in the reactivity series (carbon isn’t in the metal reactivity series but it would be right above zinc so think of it as “for a metal above zinc in the reactivity series“), the method of extraction is electrolysis.
This is because they cannot be reduced by carbon at reasonable temperatures as the metals are more reactive than Carbon and therefore Carbon cannot take away the oxygen from the metal oxide
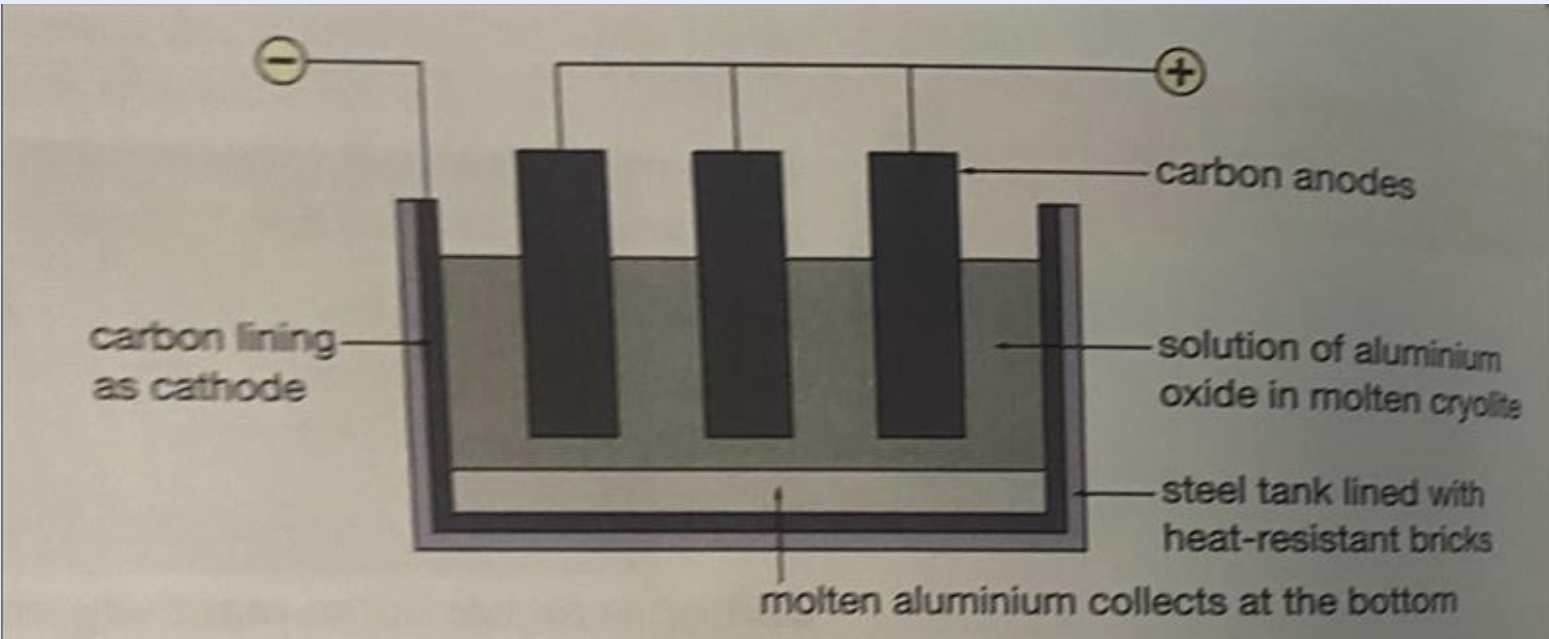
Aluminium is a good example for this. Aluminium is extracted by the electrolysis of aluminium oxide (Al₂O₃) dissolved in a molten salt called cryolite. (Look at the attached image for a diagram)
Electrolysis requires a lot of electricity, which makes it expensive. This is why a metal such as aluminium, which has to be extracted through electrolysis, is more expensive than one like iron, which is extracted by reduction with carbon.
Note that iron and all other metals (below zinc/carbon) could also be extracted by electrolysis but why would we when there’s a cheaper option?
Other extraction methods (idk if you even need this)
Not really a full method but if you have a compound, e.g. a sulfide, you can heat it by air in the process roasting to convert it into an oxide
Some metals, such as titanium, are extracting by heating the compound with a more reactive metal. This is expensive because you have to first extract this more reactive metal.
Alloys
An alloy is a mixture of metal with, usually, other metals or carbon.
E.g. steel is an alloy of iron with carbon. Brass is an alloy of copper and zinc.
Other examples of alloys: Bronze is an alloy of copper and tin. Stainless steel is an alloy of iron with chromium and usually nickel. Cupronickel is an alloy of copper and nickel (it’s used to make “silver“ coins)
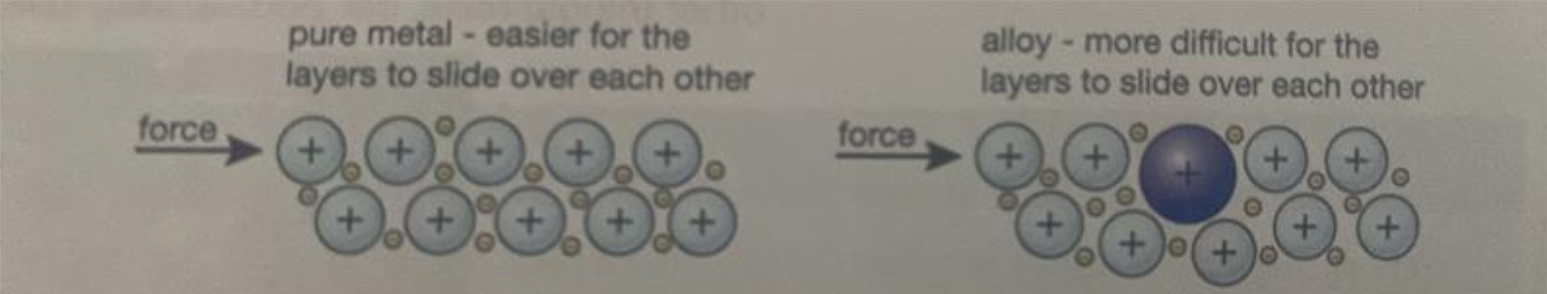
Why are alloys harder than pure metals?
In an alloy, the different metals/elements have slightly different sized atoms. This breaks up the regular lattice arrangement (in metallic bonding) and makes it more difficult for the layers of ions to slide over each other.
Look at the diagram attached.
Uses and properties of aluminium
Pure aluminium isn’t very strong so aluminium alloys are often used instead. The aluminium can be strengthened by adding other elements such as silicon, copper or magnesium.
Planes
Electricity cables
Car bodies
Pots and pans
These uses depend on its low density and strength (when alloyed), its ability to conduct electricity and heat, and its ability to resist corrosion.
Aluminium resists corrosion because is has a very thin, but very strong, layer of aluminium oxide on the surface. This prevents anything else reaching the surface from reacting with it.
Uses and properties of mild steel
Mild (low-carbon) steel is an alloy of iron containing around 0.25% of Carbon. The small amount of carbon increases the hardness and strength of the iron.
Mild steel is very malleable and ductile.
Nails
Car bodies
Ship building
Bridges
Girders
However, mild steel rusts when exposed to oxygen and water. It’s also denser than aluminium, so if a car body was made from aluminium it wouldn’t rust.
Uses and properties of high-carbon steel
High-carbon steel is an alloy of iron containing about 0.6-1.2% carbon.
It is harder and more resistant to wear than mild steel but more brittle (not as malleable and ductile).
Cutting tools (scissors, knives)
Masonry nails
Uses and properties of stainless steel
Stainless steel is an alloy of iron with chromium and often nickel.
Chromium forms a strong oxide layer which protects the iron. Stainless steel is therefore very resistant to corrosion.
Cutlery
Cooking utensils
Kitchen sinks
Gardening tools
Stainless steel is more expensive than mild steel
Uses and properties of copper
Electrical wires - good conductor of electricity and is ductile
Pots and pans - Good conductor of heat, quite unreactive and malleable
Water pipes - malleable, quite unreactive so won’t react with hot or cold water
Surfaces in hospitals - Malleable and antimicrobial properties