Properties of Amines
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What is a base?
A proton acceptor (H+).
Explain how amines are able to act as bases.
The nitrogen atom has a lone pair of electrons which allows amines to accept protons by a co-ordinate bond.
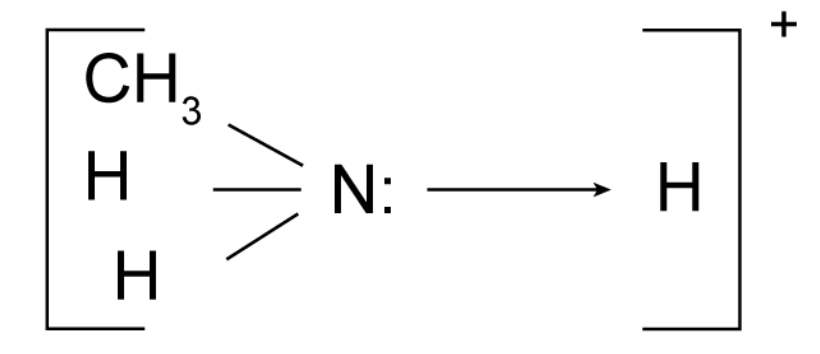
Are amines weak or strong bases?
Amines are weak bases.
What determines how strongly an amine is able to act as a base?
The strength of the base depends on the availability of the lone pair of electrons on the nitrogen atom.
The higher the electron density, the more readily available to lone pair of electrons are.
The electron density on the nitrogen depends on the type of group attached to the nitrogen.
State the order of basic strength for the following compounds (from least to most):
Ammonia
Aliphatic amines
Aromatic amines.
Least basic: Aromatic amines
Ammonia
Most basic: Aliphatic amines
Explain why aromatic amines are weaker bases than ammonia and aliphatic amines.
Aromatic amines contain a benzene ring.
Benzene is an electron withdrawing group- the lone pair of electrons on the nitrogen atom is drawn into the delocalised π-electron system of the benzene ring.
The electron density reduces; this means the lone pair of electrons is ‘less available’ and therefore less likely to accept a proton.
Explain why aliphatic amines are stronger bases than ammonia and aromatic amines.
Alkyl groups are electron pushing groups- they push the lone pair of the electrons towards the nitrogen atom.
The electron density around the N atom increases so the lone pair of electrons is ‘more available.’
Lone pair is more likely to accept a proton, making aliphatic amines stronger bases.
Describe what happens to the boiling points of amines as the chain length increases and explain why.
Boiling point increases- there are more VDW forces between the molecules.
Explain why amines have higher boiling temperatures than alkanes of similar relative molecular masses.
Amines can form both hydrogen bonds and VDW forces between molecules; alkanes can only contain VDW forces.
How can amines form hydrogen bonds?
Hydrogen bonds form between the lone pair of electrons on the electronegative nitrogen atom in one molecule and the slightly positive hydrogen atom in another molecule.
Explain why amines have lower boiling temperatures than alcohols.
Nitrogen is less electronegative than oxygen, so the hydrogen bonds between the amine molecules are weaker than those between alcohol molecules.
Are amines soluble in water?
Yes- amines are soluble in water as they can form hydrogen bonds with water molecules.
Why is phenylamine only slightly soluble in water?
Due to the hydrophobic nature of the benzene ring.