Galvanic Cells - AOS 1
1/44
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
* O: -2
* F: -1
* Halogens (group 7): -1, +1 with oxygen
Conjugate pairs
Reducing agent = conjugate oxidising agent
Oxidising agent = conjugate reducing agent
strong reducing agents have weak conjugate oxidising agents
2\. Balance hydroxide ions by adding OH-
* galvanic cell = spontaneous
* electrolytic cell = non-spontaneous
* exothermic reaction process
* occurs in all fuel cells
If a cell contains only non-solid materials or if an electrode is aq then…
an inert electrode (e.g: platinum or graphite) is used
cell potential difference
E0 (oxidant) top → E0 (reductant) bottom
cell voltage must be positive
if negative, need an external power source
primary cell
galvanic cells
non-rechargeable
spontaneous
products slowly migrate away from electrodes or consumed by side reactions preventing recharge
go flat because reactants get used up → voltage or current drops
has to go through an external cell with two half-cells separated
salt bridge
energy transformation:
- during the discharge cycle, products build up around the electrodes and slow down or even stop the discharging process (polarisation)
- when the cell is allowed to rest, the accumulated products move away from electrodes, allowing for further redox reactions
e.g: daniel cell, alkaline cell
secondary cell
electrical energy is converted to chemical energy
non-spontaneous
galvanic cell in discharge (using = galvanic), electrolytic cell on recharge
rechargeable battery
reaction can be forced backwards to recharge cell (anode +, cathode -)
energy transformation:
- when it discharges, it acts as a galvanic cell, converting chemical energy to electrical energy
- when it recharges, it acts as a electrolytic cell. Electrical energy is transformed into chemical energy
e.g: lithium cell, lead acid battery
fuel cells
A type of galvanic cell that converts chemical energy into electricity and requires a constant supply of reactants to be added
exothermic reaction
cannot be recharged
e.g: hydrogen fuel cell
40-60% efficient
do not have direct contact between oxidant and reductants
safety issues with hydrogen:
- flammable and highly combustible, low density which means that it rises rapidly and will quickly disperse in fire
fuel cells characteristics
hydrogen fuel cell only produces water = sustainable
electrodes must be porous and conducting
increasing amount of electrical energy produced can improve efficiency (e.g: increasing surface area or using a catalyst as electrode) → anode = platinum / cathode = nickel powder
porous electrodes are more efficient at moving charges and reactants
larger surface areas = more amount of electrical energy
advantages of hydrogen fuel cells
converts chemical → electrical energy
sustainable, doesn’t produce greenhouse gases
more efficiency → requires less energy conversions
high energy density of H2 means less fuel needs to be carried
* needs a constant supply of fuel
* storage can be an issue as H2 is FLAMMABLE/EXPLOSIVE
advantages and disadvantages of generic fuel cells
advantages
- convert chemical energy directly to electrical energy → more efficient than the series of energy conversions that take place in power stations that burn fossil fuels
- generate electricity as long as fuel is supplied (does not need to be recharged/replaced)
disadvantages
- expensive compared to conventional fuels, platinum catalyst for example is non-renewable
**Fuel:** anode, cathode, porous electrode, charges are carried in electrode
**Fuel:** 40-60%
**Fuel:** large vehicles, cars, buses
functions of a membrane (hydrogen fuel cell)
keep the O2 and H2 gas separated
prevent spontaneous reactions between products
hydrogen gas fuel cells equations (alkaline)
Oxidation (anode) : H2 + 2OH- → H2O + 2e- (hydrogen is oxidised)
Reduction (cathode): O2 + 2H2O + 4e- → 4OH- (oxygen is reduced)
energy transformations
discharge:
- oxidation occurs at anode (-)
- reduction occurs at cathode (+)
recharge (swap charges):
- oxidation occurs at anode (+)
- reduction occurs at cathode (-)
Primary/Secondary Cells vs Fuel Cells*
Definition
- Primary or secondary cell is a type of galvanic cell. These galvanic cells are electrochemical cells that convert chemical energy from spontanteous redox reactiosn into electrical energy
- A fuel cell is a galvanic cell that converts the chemical energy of a fuel into electrical energy. Air or oxygen are supplied continuously
Function
- A primary cell is a source of portable electrical energy
- Fuel cell is a continuous source of high electrical current for both portable and fixed applications
Features
- Fuels cells have reactants that are contained within the cell. Thus, can produce power for only a limited time until their reactants are depleted
battery life
loss of active materials (reactants and products)
products detach from electrodes
side reactions
impurities in cell materials
corrosion or failure of internal components
temperature: batteries sensitive to heat, low temps help to prevent self-discharge but rate of reaction will fall at low temps
Similarities & Differences - Fuel Cells & Seconadry Cells
differences
- fuel cells are continuously supplied with fuel and oxygen
- secondary cells can only produce power up until their reactants are depleted
similarities
- both convert chemical energy into electrical energy
- anode (oxidation) and cathodes (reduction)
why do electrodes have to be porous
- porous electrodes maximise surface area and increases the rate of reactions at the anode and cathode (allows reactants to come into contact with electrolyte)
- this results in a higher current because of the higher rate of reaction
- the cell voltage, however, is independent of reaction rate and will remain the same
inert electrodes examples
platinum, gold, graphite(carbon), and rhodium
fuel cells - electrodes & electrolytes
- must be conductive
- must be porous to allow the reactants to come into contact with the electrolyte
- larger surface area = higher amount of energy
- catalyst (anode = platinum / cathode = nickel powder)
fuel cell design (hydrogen-oxygen)
- two seperate reactant compartments and an electrolyte solutions
- separated from the electrolyte by porous electrodes
anode: hydrogen
cathode: oxygen
industrial applications
molten electrolytes
- higher energy expenditure, greater wear on cell
- expensive, lots of energy tp keep ions
aqueous electrolytes
- water will undergo oxidation and reduction and so may react preferentially to the cations and anions present from salt
- cheaper
electrorefining
used for purifying metals
- metal containing impurities is used as the anode
- pure metal is placed at the cathode
- any metals (impurities) that are stronger reductants will be oxidised at the anode
- any wear instead fall on the bottom of cell (anode mud)
electroplating
process by which a thin layer of metal is deposited onto a metal electrode (electrolysis)
- prevents corrosion
process:
- object plated is attached to the negative terminal (cathode), it is then placed in an electrolyte solution containing pure ions of the metal that forms the plating
- anode will form the plating onto cathode
- power supply removed electrons from positive electrode (anode) and gives them to cathode (negative electrode)
alkaline fuel cell
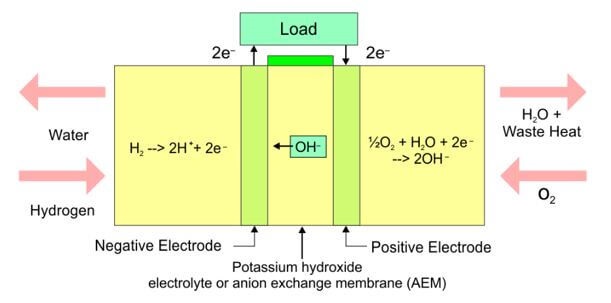
explain why the two half-cells are seperated in fuel cells
The half-cells need to be separated so that electrons can travel between the two half-cells in connecting wires (through the load) and the electrical energy harnessed.
If there was no separation of the two half-cells, oxidation and reduction would occur together and electrons would not move through the external circuit.
state the feature that causes the overall movement of H+ ions across the membrane
At the cathode the [H+] decreases and at the anode the [H+] increases. The H+ ions move from the higher concentration through the membrane to the lower concentration.
a high concentration in the presence of water means that
it will be the strongest oxidant/reductant
factors that limit the life of a fuel cell
- side reactions at the electrodes
- significant temperature change
- build-up of gases around electrode
characteristics and functions of cells
electrolyte: carry current between the electrodes by the movement of cations (+ ions) towards the cathode and anions (– ions) towards the anode
electrode: transport produced electrons from one half-cell to another, which produce an electrical charge
membrane: keep the O2 and H2 gas separated prevent spontaneous reactions between products
safety considerations of hydrogen fuel cells
- controlling possible ignition sources since hydrogen is highly flammable and explosive in the presence of a spark in air
- following appropriate hazardous materials guidelines
- safe storage/location of hydrogen containers
what temperatures is suitable for batteries
room temperatures (non-extreme)
advantages of batteries
- efficient, less waste of energy/fewer products released to atmosphere
- oxygen not requires