Instrumental Midterm Review
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
101 Terms
Explain Ohms law
Relationship between voltage, resistance, and current in a resistive series current. V=IR, where V is the potential difference between two points in a circuit in volts, I is the resulting current amperes in amperes, and R is the resistance between points in ohms.
Explain Kirchoffs Current Law
States that the algebraic sum of currents at any point in the circuit is 0, so the sum of currents going in must equal the sum of currents going out.
Explain Kirchoffs Voltage Law
States that the algebraic sum of voltage in a closed circuit is 0, so the sum of the voltage increase must equal the sum of voltage decrease - conservation of energy.
Explain Direct Current Circuit and name its types
Direct current (DC) is the flow of electric charge in only one direction, from negative to positive. It is the steady state of a constant-voltage circuit. The types are series resistive and parallel resistive
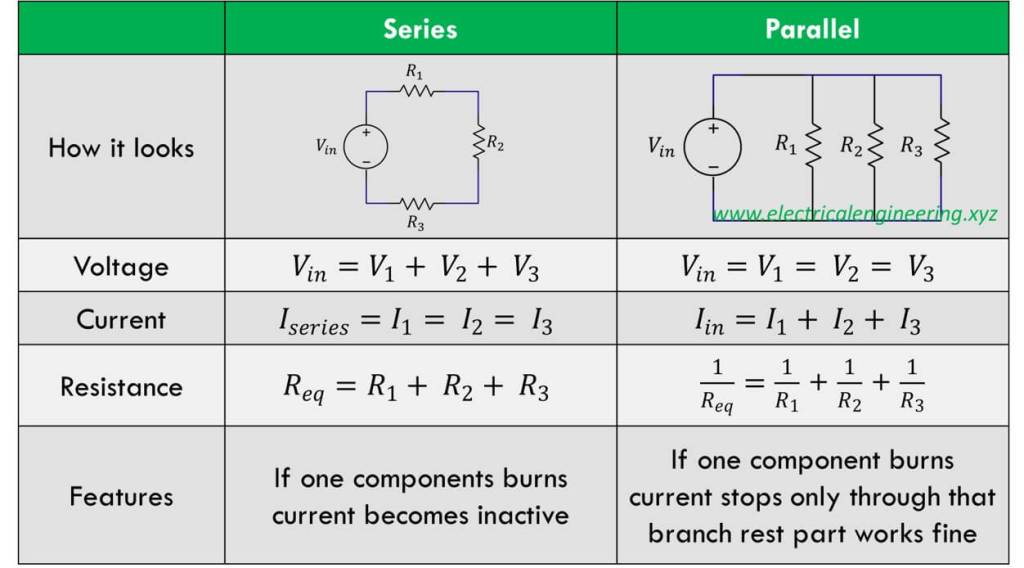
Explain Series Circuit
Series circuit: the components are connected in a line. The total resistance is the sum of the individual resistances, the current remains the same throughout, and voltage drops across each resistor (based on its resistance)
Explain voltage divider
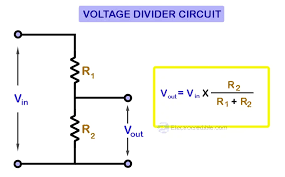
It is a sample circuit that divides the input voltage into smaller fractions using resistors, so that the voltage across one of the resistors in a series circuit can be calculated.
Fraction of the total voltage appears across each resistor. Voltage dividers are widely used in electrical circuits to provide output voltages that are a fraction of an input voltage. In this mode, they are called attenuators and the voltage is said to be attenuated.
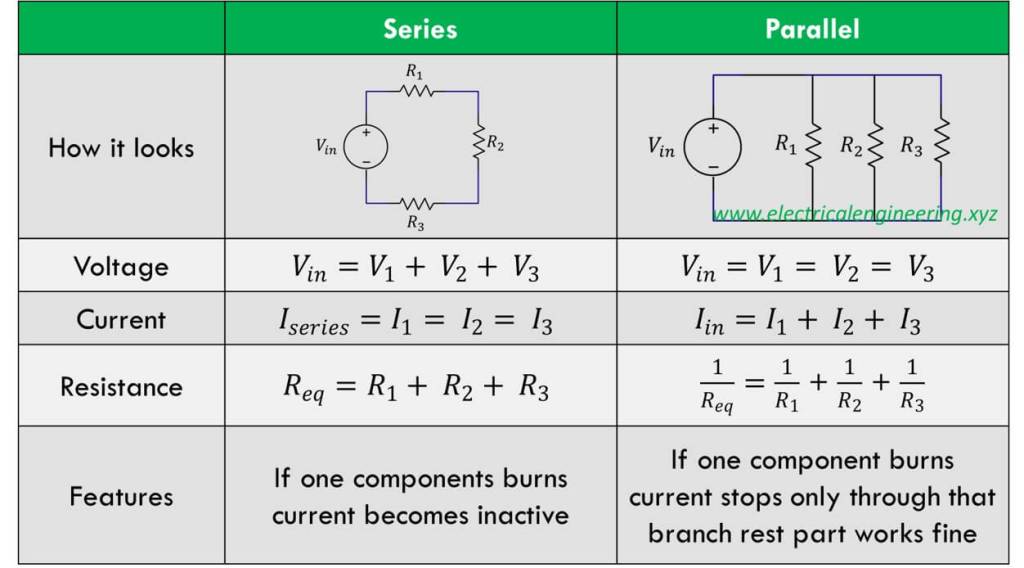
Explain parallel circuit
The components are connected so that each component has its own separate branch and the same voltage is applied to each component, specifically each resistor gets the same amount of voltage. The total resistance is the reciprocal of the sum, the voltage is the same for each resistor, and the current divides between the resistors depending on their value.
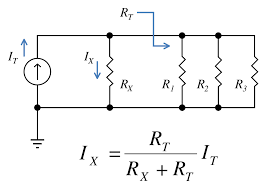
Explain Current Splitter
Parallel resistors create a current divider, or splitter. For two parallel resistors, the fraction of the current in one resistor is just the ratio of the resistance of the second resistor to the sum of the resistances of the two resistors.
Explain analog to digital converter (ADC)
It is the heart of the integrated circuit which converts the input analog signal to a number that is proportional to the magnitude of the input voltage.
Explain alternating current
Electrical charge flows back and forth, changes direction. If it is expressed as the root-mean-square, or rms, value produces the same heating in a resistor as a direct current of the same magnitude.
What is an inductor?
It resists the change in current as energy is stored in the magnetic field of the inductor, it is written as a a coil of copper wire in Skoog but there may be different types.
What is a resistor?
A resistor restricts the flow of current, and it is inversely proportional to temperature.
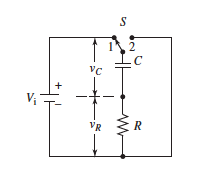
What is a capacitor?
A capacitor in an AC circuit resists changes in voltage. It consists of a pair of conductors separated by a thin layer of a dielectric substance—that is, by an electrical insulator that contains essentially no mobile, current-carrying, charged species. A useful property of a capacitor is its ability to store an electrical charge for a period of time and then to give up the stored charge when needed. Picture explains how it works.
What is reactance?
It is the resistance of inductors to changes in current and the resistance of capacitors to changes in voltage.
What are the types of reactance and what are their relationships to frequency?
The two types of reactance capacitive reactance and inductive reactance, both of which depend on frequency. At low frequency when rate of current change is low, then inductive reactance is low (proportional relationship). Capacitive reactance is largest at low frequencies (inverse relationship). These reactances are important for converting AC to DC, discriminating among signals of different frequencies, separating ac and dc signals, and differentiating or integrating signals.
What are nonlinear devices, what do they do, and name some types?
The input and output voltages or currents of nonlinear devices are not linearly proportional to one another, so they are used to change an electrical signal from ac to dc (rectification) or the reverse, to amplify or to attenuate a voltage or current (amplitude modulation), or to alter the frequency of an ac signal (frequency modulation).
Some types are transistors, semiconductor diodes, and vacuum or gas-filled tubes. Semiconductor-based diodes and transistors are used more due to low cost, low power consumption, small heat generation, long life, and compactness. Now integrated circuits are used as they have many as a million transistors, resistors, capacitors, and conductors on a single tiny semiconductor chip.
What is a semiconductor?
A semiconductor is a crystalline material with a conductivity between that of a conductor and an insulator. There are many types of semiconducting materials, including elemental silicon and germanium; intermetallic compounds, such as silicon carbide and gallium arsenide; and a variety of organic compounds- most common are crystalline silicon and germanium.
Picture explains how it works. The first part is “Silicon and germanium are Group IV elements and thus have four valence electrons available for bond formation. In a silicon crystal, each of these electrons is localized by combination with”
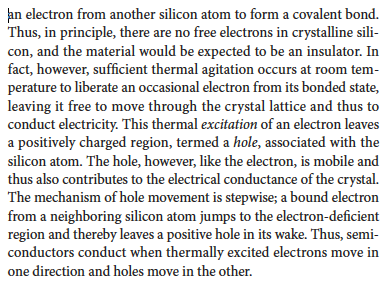
How can the conductivity of a crystalline silicon/germanium be enchanced?
By doping which is when a tiny amount of a Group V or III element is introduces by diffusion that adds an unbound electron to the lattice structure, so the thermal energy needed to free it is small.
More info in the picture.

What is a semiconductor diode?
A diode is a nonlinear device that has a greater conductance in one direction than in the other, formed with adjacent n and p type regions - the interface between them is the pn junction.
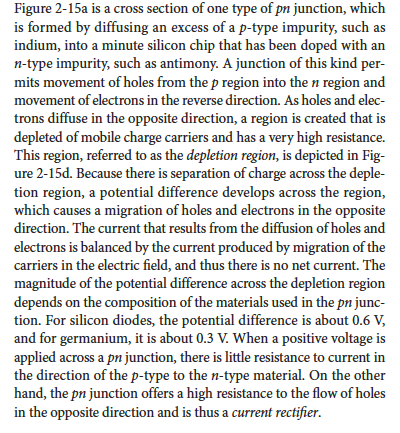
What is a pn junction?
Controls the flow of current, where it passes easily in one direction and is resisted in the other. More info in the picture.
What is a transistor?
Basic semiconductor amplifying and switching device.
What is a transformer?
Increases or decreases the voltage from an AC power.
Explain signal and noise, and the ratio.
Signal is the information about the analyte. Noise is made up of unwanted fluctuations in the signal that obscure the measurement and degrade precision and accuracy.
The signal to noise ratio measures how distinguishable the signal is to noise, so higher S/N ratio means the signal is more distinguishable resulting in better analytical accuracy. The ratio is the mean of the signal over the standard deviation of the noise, or reciprocal of relative standard deviation.
What are the two types of noise sources? Explain.
Chemical and instrumental.
Chemical are uncontrollable chemical variables such as fluctuations in temperature, pressure, or concentration. These affect equilibrium, reaction kinetics, and physical properties which affect the measured signal. Laboratory conditions also create noise due to humidity or light intensity.
Instrumental are due to component imperfections of the instrument, and there are four types: Thermal, Shot, Flicker, and Environmental. Thermal noise results from the random movement of electrons in resistive materials which increase with temperature. Shot noise is caused by the quantized nature of electron movement across junctions. Flicker noise, also known as one over f, occurs at low frequencies and is inversely proportional to frequency. Environmental noise is caused by external factors like electromagnetic interference from radio waves or power lines.
Explain the types of hardware devices used for noise reduction.
Grounding and Shielding reduces electromagnetic interference. Grounding provides a safe path for excess electricity and shielding protects the circuitry from external interference by surrounding it with a conductive material.
Difference and Instrumentation Amplifiers: difference amplifiers attenuate noise caused by the transducers as they are usually amplified. They reject noise common to both input signals, known as common mode noise. Instrumentation amplifiers improve signal clarity by only amplifying the differential signal.
Analog/Low-Pass Filtering: filter allows low frequency signal to pass while attenuating high frequency noise.
Modulation converts a low frequency signal to high frequency to reduce flicker noise.
Explain the types of software methods used for noise reduction.
Ensemble averaging improves the S/N ratio by averaging multiple scans of signals, and since noise is random it tends to cancel over multiple measurements but the signal remains consistent, leading to a clear signal.
Boxcar Averaging smooths the data by averaging a set number of adjacent data points and replacing the central data point with the average.
Digital filtering can be applied to remove specific frequencies from a signal, such as high frequency noise. An example is Fourier transform which are used to convert from time to frequency domain which filter unwanted frequency components and allow for easy identification. (Fourier transform used in IR, specifically fast Fourier transform)
Least Squared smoothes noisy data by applying a weighted average to adjacent data points which minimizes noise without harming the signal.
Correlation methods compare signals with references to eliminate noisy data.
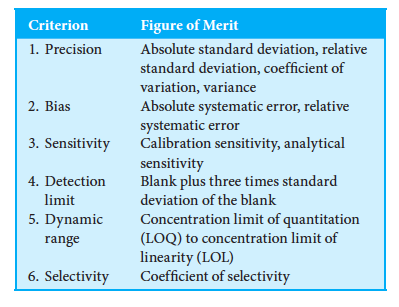
Explain the figures of merit and its criterion.
Precision: Degree of mutual agreement among data that have been obtained (how close your experimental values are to each other). It provides a measurement of the random, indeterminate error of analysis using absolute and relative standard deviation, standard error of the mean, coefficient of variation, and variance.
Bias (Accuracy): Procides a measure of the systematic, or determinate, error of a method (how far your experimental value is to the true value, accuracy). It is found by doing an absolute and relative systematic error. (analyte concentration population mean-true value)
Sensitivity: measure of the instruments ability to discriminate between small differences in analyte concentration. Two factors limit sensitivity the slope of the calibration curve and reproducibility of the device. A steeper slope will be the more sensitive than a linear one, but this factor does not take the precision of individual measurements into account. Reproducibility is method based and it is based on the idea that if the method was done at two different places, with different people and equipment then a similar result would found.
Detection Limit: minimum concentration or mass of analyte that can be detected AT A KNOWN CONFIDENCE LEVEL. Depends on the ratio of the magnitude of the analytical signal to the size of the blank signal’s statistical fluctuations. Thus, the analytical signal has to be larger by the blank by a variable to be detected. Ingle says the variable should be three based on t and z tests. He states that the blank data should not be assumed to be normal and at 3 times the standard deviation of the blank the confidence level will be 95%.
Dynamic Range: An analytical method should have a few orders of magnitude, but that depends on techniques (absorption spectrophotometry have 1-2). Dynamic range is based on lowest concentration at which quantitative measurements can be made (LOQ) to the concentration at which the calibration curve departs from linearity (LOL). Usually, a deviation of 5% from linearity is considered the upper limit. Deviations from linearity are common at high concentrations because of nonideal detector responses or chemical effects. The lower limit of quantitative measurements is generally taken to be equal to ten times the standard deviation of repetitive measurements on a blank. At this point, the relative standard deviation is about 30% and decreases rapidly as concentrations become larger.
Selectivity: Degree to which an analytical method is free from interference by species in the matrix (ability to discriminate between small differences and concentration). Based on coefficient of selectivity, which can range from zero (no interference) to values considerably greater than unity. Note that a coefficient is negative when the interference causes a reduction in the intensity of the output signal of the analyte.
Specificity
Tests that are specific to one thing
Want to identify a specific thing
When you see something red, don’t assume its blood
Potato starch might set off some tests
Limit of reliability
Consistently hitting detection threshold
Getting same measurement each time
Traceability - ability to backtrack where the signal comes from
Able to backtrack chemicals/solvents through identifiers
Ruggedness - can be used at varying parameters
What are the characteristics of a normal distribution:
Mean, median and mode are equal
Symmetrical
What is the difference between population and sample?
Population: approaches infinity
Sample: Subset of the population
What is a Q test?
Used to reject values
Calculated by (gap)/range, where gap is the value in question - the one nearest to it
If your calculated Q is higher than the standard Q value, then you reject
Number of observations = how many values in sample
What is a confidence interval?
With confidence, the true value lies within the range of the observed value within absolute error
Describes the amount of uncertainty associated w/ a sample estimate of a population
More participants = smaller confidence interval
Parameters of the population are estimated based on a sample in statistics
Parameters that are estimated are the mean or variance etc
Mean of the sample if most likely different from the mean of the population with each sample showing different means
Know the range of which the true value lies with a high probability
This range is called a confidence interval
Range b/w lower and upper levels is the confidence interval
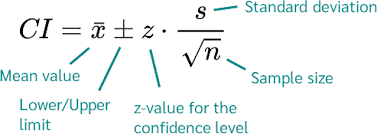
Explain T test.
To see if certain values are within a certain range
Instead of 1 data set being tested, its two
Take samples from different populations (ex. Taking 2 pieces of barley from Two fields of barley)
Ratio of signal to noise
Signal is the difference b/w group means
Calculate means in each group and find the absolute value
Noise is the variability of the groups
Calculated w/ variance (sd2)
Increasing number of samples increases the signal to a point -> causes higher T value
If T value is higher than 1, there’s more signal than noise
H0 is a null hypothesis - there’s no statistically significant dif b/w the samples
Use a table w/ degrees of freedom to find critical values in order to see if you reject the suspected value or not
If t value is higher than critical value, null hypothesis is rejected which shows that there is something statistically significant b/w two sets
Degrees of freedom = n1 + n2 - 2
Assumptions
Normal distribution in populations and sample sizes
Roughly same number of data points in each sample
Want to be in 20-30 point range for sample

How is a UV-Vis chart normally displayed? What are the axes?
A UV-vis spectra has an x axis of wavelength in nm and a y axis of absorbance
How is absorbance determined from what the instrument actually measures?
Absorbance is how much light the sample absorbs and its measured by taking the -log of transmittance, transmittance is the ratio of the radiant power to the radiant power after going through the sample.
What is the Beer Lambert Bouguer Law (Beer's)? Why is it important? Define the factors. How is each determined or evaluated?
Beers law is the mathematical relationship between absorbance, concentration, path length, and molar abs (A=ebc) , it is important as it helps in qualitative and quantitative measurements. Its factors are:
the amount of absorbing material in its path length,(concentration)
the distance the light must travel through the sample (pathlength)
the probability that the photon of that particular wavelength will be absorbed by the material (absorptivity or extinction coefficient).
Absorbance is given by the instrument, pathlength is the distance between the cell walls, concentration is the amount of analyte in a solution, and molar absorptivity is found by doing a calibration curve
What are the limits of Beers law?
when b is constant there may be proportionality deviations between abs and conc - real deviations-, how abs measurements are made -instrumental deviations-, or chemical changes due to conc -chemical deviations.
Real: At high conc, the molecules and ions responsible for abs have a diminished avg distance that causes the particle to affect its neighbors, which are solute-solute interactions- which affects the absorbance at a certain wavelength. Moreover, absorptivity depends on the refractive index of the medium, if conc changes makes changes in a solution's refractive index, deviations occur.
Chemical deviations can occur when an analyte dissociates, associates or reacts with a solvent to produce a product with diff absorbing properties, seen in acid base indicators where color changes when there is a shift in equilibrium.
Instrumental deviations: Stray radiation caused by scattered radiation- due to reflection or scattering from optics -from the outside that is not the needed wavelength will cause deviations at high conc and longer paths. Moreover, Mismatched cells cause deviations if blank and analyte path lengths and optical characteristics are not identical as it will cause an intercept in beers law.
What is the purpose of the Holmium Oxide or Didymium standard?
It validates the wavelength scale of UV-VIS, verifies calibration.
In order to determine the % or concentration of an unkown by photometry, what is necessary for you to have (besides an instrument)?
Identify unknown then make solutions of varying concentrations then record their absorbance. Then, make plot the data (absorbance as a function of concentration), and the best fit line will be beers law, with the slope being molar absorptivity. Then, measure the unknowns absorbance and place into the calculated beers law from the best fit line. To verify the calibration curve, make a known solution and measure the absorbance and compare to the best fit value.
What is a chromophore?
Chromophores are the functional groups that absorbs UV light at a certain wavelength, could have color or be colorless.
What happens on the molecular level that gives rise to the spectra?
The energy from the source at a particular wavelength will have enough energy to excite an electron from its rest, ground state to a higher energy level; this is done as the electron absorbs the light photon. The higher energy level is unstable so the electron degrades to its ground state, but because the electron absorbed a light photon, the detector registers a lower energy/ intensity.
What is the purpose of the part of the experiment in which four cuvette materials are used?
The four cuvettes are made of different materials which will have different effects on transmission.
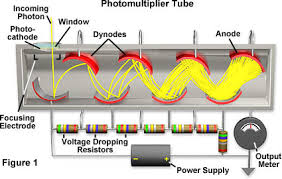
Explain UV-Vis detector.
The detector is a photomultiplier tube. The photon hits the photocathode that converts photons to electrons via photoelectric effect where photons hit the material at enough energy to release an electron. The electron hits a dynode which are high voltage electrodes - high voltage indicates strong electric field. When the electron hits the dynode, more electrons release and travel to the next dynode which is higher in voltage, thus more electrons release; this is also caused due to increased acceleration, at each dynode there is a stronger force so more electrons are pushed out. This continues until the anode which is connected to a wire that transmits an electric signal.
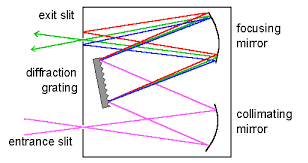
Explain UV-Vis dispersing unit.
The dispersing unit is a monochromator which is used to separate and select wavelengths. The light enters through the entrance slit which allows a portion of the light through then goes to the collimating lens which makes the light into a column, parallel beam. The beam then hits the dispersing unit- which could be a prism or grating- that separates the beam into its wavelengths, then it hits the focusing mirror to focus it onto the exit slit. The exit slit allows a certain wavelength through.
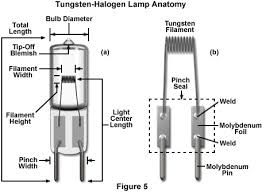
Explain UV-Vis source.
The source is a continuum source, the tungsten halogen or deuterium. Tungsten halogen heats up due to current, and the highly resistive elements lose resistance allowing for electron movement causing light. The bulb is filled with halogen gas, and when the material deposits, instead of depositing on the bulb it bonds with the halogen to create a complex that when cooled disassociates and the material redeposits on the filament. This allows the tungsten halogen to have increased lifetime and can be used at higher temperatures leading to more light. Deuterium is used as
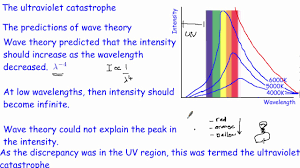
What is the UV catastrophe?
Energy was theorized to infinitely increasing, but it was found that energy is quantized, meaning that energy is released in packets/chunks/groups.
Why were you asked to prepare two forms of the papaverine (or drug that the tech supplies) in the KBr pellets? What are you looking for in the generated spectra? Why?
The forms are free organic and salt form. The salt form is the ionic form caused by the bond of positively and negatively charged atoms. The free form is the organic molecule .
What is the IR chart plot called?
The chart plot is called a spectrum which plots transmittance as a function of wavenumber in inverse cm.
What types of solvents are not compatible with most salt plates?
Polar solvents.
Name four sample preparation methods.
Pressed pellets usually KBr
Liquid cells
Mulls
Salt plate
How would you clean the salt window sealed solution cells (liquid) cells? What about salt plates?
Clean with nonpolar solvents and lens tissue, not with water, as salts dissolve in polar specifically water, damaging the cell.
Salt plates are cleaned with methylene chloride/acetone/ lens tissue.
Why do you run a polystyrene film first?
Polystyrene verifies the frequencies on a spectrometer, verifies calibration.
Why does the background have the approximate bell shape? (Disregard the sharp 'noise' that appears). Why is the transmission line not flat?
The background has a bell shape as the source is a blackbody which is an object that emits and absorbs radiation, and the curve/spectra is a blackbody. The transmission line is not flat because its the wavelength of the environment.
Why are halide salt plates employed to hold samples?
The ionic bonds cannot be read on an IR due to the bonds beacuse only covalent bonds vibrate.
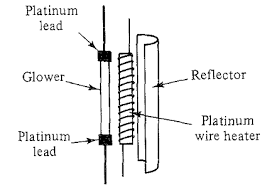
Explain IR source.
The possible sources are nernst and globar. Nernst a cylindrical rare eath dioxide that has a resistive heating element thus when the temperature increases due to the passage of a current, light is emitted. Nernst has large negative temp coefficient that it must be heated externally to a red heat before the current is large enough to be maintained The globar is a ceramic rod of silicon carbide or composite material that heats to more than 1000K, works the same as nernst, but in FTIR globar can be electronically controlled for higher stability due to its positive coefficient.
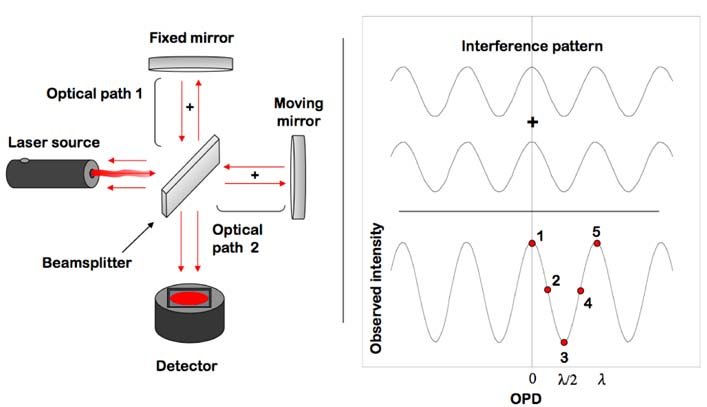
Explain IR dispersing unit.
It does not have a dispersing unit, it uses the fourier transform (FT), most common is a Michelson interferometer. The beam hits the beamsplitter(a coated plate that half transmits the beam and half reflects it made of KBr and Germanium)that splits it into two beams, one hits an immobile mirror while the other hits a mirror with constant velocity and known position. The moveable mirror causes a path difference resulting in interference so it is recorded as amplitude as a function of time, but fourier transform is a mathematical model that transforms from a time to frequency domain which help with signal to noise ratio.
Explain IR detector.
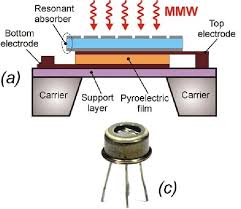
Pyroelectric detectors are made from a crystalline pyroelectric material which are dielectric (material that possesses high resistivity, but this changes when it’s heated) insulators with special thermal and electrical properties, by adding IR radiation the temp changes causing a difference in charge distribution in the crystal which is read as a current to the electrical circuit around the capacitor system. The temperature increases causes resistance to decrease allowing electron movement which is read as a current. Skoog compared it to the thermal sensitive detector
Explain the theory for IR, UV, and GC.
IR is used to identify the functional groups in a sample based on the energy changes due to molecule vibration and rotation. To absorb IR a dipole -determined by charge difference and distance between two energy sources- change has to occur. the dipole change allows interaction with the alternating field of the radiation. a change in the amplitude in the vibration occurs when the frequency of field and molecule are the same and thus is absorbed. there is no net change when homonuclear species so no IR absorption eg O2 . vibrations are either stretching or bending: stretching is changes across the distance on the axis and bending is change in bond angle and has four types: scissoring rocking wagging and twisting . stretching is similar to spring behavior as in simple harmonic oscillators
UV is used for the quantitative determination of a large number of inorganic, organic, and biological species. This is found by measuring absorbance then using beers law to calculate concentration. The energy from the source at a particular wavelength will have enough energy to excite an electron from its rest, ground state to a higher energy level; this is done as the electron absorbs the light photon. The higher energy level is unstable so the electron degrades to its ground state, but because the electron absorbed a light photon, the detector registers a lower energy/ intensity.
GC is allow the separation, identification, and determination of closely related components of complex mixtures. The components of a vaporized sample are separated as a result of differential distribution between a mobile gaseous phase and a liquid or a solid stationary phase held in a column. In performing a gas chromatographic separation, the sample is vaporized and injected onto the head of a chromatographic column. Elution is brought about by the flow of an inert gaseous mobile phase. The separation is based on solubility, the rule “like dissolves like“ states that compounds with the same solubility will interact, thus a polar stationary phase will interact with a polar compound causing it to stay in the column longer than nonpolar compounds, and the same for polar compounds.
Explain what injection splitting is and why it is necessary to use in this and all experiments.
A sample splitter is often needed to deliver a small known fraction (1:50 to 1:500) of the injected sample, with the remainder going to waste. Commercial gas chromatographs intended for use with capillary columns incorporate such splitters, and they also allow for splitless injection to improve sensitivity or for use with packed columns.
What are adjusted and corrected retention data? Why are they important? (See textbook and other references if needed) Corrected data is of less interest today. Why?
The length of time between injection and position of the target compound peak a retention time.
On the other hand, the time difference between the peak of an unretained compound and a target compound is called the adjusted retention time.The adjusted retention time is the time an analyte spends in the column not the stationary phase.
corrected is
Do you expect peak shapes (not size) to be the same for all the standards on column A? Assume column A is non-polar such as polymethyl siloxane. Explain.
Ideally, they should display a Gaussian bell-shaped curve. However, we might observe tailing or fronting with the chromatograms. The typical Gaussian shape of a chromato- graphic band can be attributed to the additive combination of the random motions of the various molecules as they move down the column. A common cause of tailing and fronting is a distribution constant that varies with concentration. Fronting also arises when the amount of sample introduced onto a column is too large.
What is programming the oven (ramping) in solvent mixture analysis by GC? What are its advantages?
Better separation, resolution, clearly defined peaks. The Column temperature is an important variable that must be controlled to a few tenths of a degree for precise work. Thus, the column is ordinarily housed in a thermostatted oven. The optimal column temperature depends on the boiling point of the sample and the degree of separation required. Roughly, a temperature equal to or slightly above the average boiling point of a sample results in a reasonable elution time (2 to 30 min). For samples with a broad boiling range, it is often desirable to take advantage of temperature programming (ramping), in which the column temperature is increased either continuously or in steps as the separation proceeds. In general, optimal resolution is associated with minimal temperature; the cost of lowered temperature, however, is an increase in elution time and therefore the time required to complete an analysis.
How will you identify the unknown solvent(s) if present? Is this or can this be a positive identification? How?
To identify an unknown, multiple standards are tested in the same columns to gather their retention time under the same conditions as the unknown. The unknown is run, and its retention times are compared to the standards.
Is there a better instrumental method for this type of analysis? What is it? What are its advantages?
First, GC is a tool for performing separations. In this role, GC methods are unsurpassed when applied to complex organic, metal-organic, and biochemical systems made up of volatile species or species that can be derivatized to yield volatile substances. The second role that GC plays is in the completion of an analysis. In this role, retention times or volumes are used for qualitative identification, and peak heights or peak areas provide quantitative information. For qualitative purposes, GC is much more limited than most of the spectroscopic methods.Thus, chromatography is often an essential step in qualitative spectroscopic analyses.
What are optical spectroscopic methods based on?
(1) absorption measures the amount of light absorbed as a function of wavelength, (2) fluorescence, (3) phosphorescence (both 2 and 3: by emission of photons is measured after absorption), (4) scattering, (5) emission usually involves methods in which the stimulus is heat or electrical energy, and (6) chemiluminescence refers to excitation of the analyte by a chemical reaction.
Measurement of absorption, fluorescence, and phosphorescence, require an external source of radiant energy. For fluorescence and phosphorescence, the source induces the sample, held in a container, to emit characteristic radiation, which is usually measured at an angle of 90 with respect to the source. Emission spectroscopy and chemiluminescence spectroscopy differ from the other types in that no external radiation source is required; the sample itself is the emitter. In emission spectroscopy, the sample container is a plasma, a spark, or a flame that both contains the sample and causes it to emit characteristic radiation. In chemiluminescence spectroscopy, the radiation source is a solution of the analyte plus reagents held in a transparent sample holder. Emission is brought about by energy released in a chemical reaction in which the analyte takes part directly or indirectly.
What are the components of a spectroscopic instrument?
(1) a source of radiant energy; (2) a container for holding the sample; (3) a device that isolates a restricted region of the spectrum for measurement2; (4) a radiation detector, which converts radiant energy to a usable electrical signal; and (5) a signal processor and readout, which displays the transduced signal on a digital display, a computer screen, or another recording device.
Explain source and its two types.
A source must generate a beam with sufficient radiant power for easy detection and measurement. In addition, its output power should be stable for reasonable periods. Thus, a regulated power source is almost always needed to provide the required stability. Alternatively, the problem of source stability can sometimes be circumvented by double-beam designs in which the ratio of the signal from the sample to that of the source in the absence of sample serves as the analytical variable. The types of source are continuum and line, continuum emit radiation that changes intensity as a function of wavelength and line sources emit a limited number of lines -bands- each of which span a limited range of wavelength.
There is also Continuous where the light is always on/constant supply
what are continuum sources used for? Name its types.
Continuum sources are used in absorption and fluorence spectrometry.
UV used deuterium lamp. If an intense source is needed then high pressure gas lamps filled with argon,xenon,or mercury are used.It is a continuum source because when the two atoms are excited they have a varied kinetic energy which leads to a variation in frequency that results in a continuum spectrum.
Vis uses a tungsten filament lamp.
IR uses inert sources that heat to 1500-2000K, a temperature at which the maximum radiant output occurs at a wavelength of 1.5-1.9 micrometers.
what are line sources used for? Name its types.
Line sources that emit a few discrete lines are used in atomic absorption, atomic and molecular fluorescence, and Raman.
Mercury and sodium vapor lamps provide few,sharp lines in the UV-Vis range.
Hallow-cathode lamps and electrodeless discharge lamps are used for atomic and fluorescence methods.
Lasers sources are used in analytical instrumentation because of their high intensities, their narrow intensities, and the coherent nature of their outputs. useful applications for these sources in high-resolution spectroscopy, in probing and monitoring ultra-fast phenomena, in the detection and determination of trace elements in Martian soil and in the earth’s atmosphere, and in forensic science. In addition, laser sources have become important in several routine analytical methods, including Raman spectroscopy, molecular absorption spectroscopy, fluorescence spectroscopy, emission spectroscopy, and as part of instruments for Fourier transform IR spectroscopy.
How do Lasers work?
Lasers is an acronym for light amplification by stimulated emission of radiation. Due to its light amplification, lasers produce spatially narrow, extremely intense beams of radiation. The heart of the laser is a lasing medium either made of solid crystal of ruby, a semiconductor of gallium arsenide, a solution of organic dye, or a gas of argon or krypton. A laser normally functions as an oscillator, or a resonator, in the sense that the radiation produced by the lasing action is caused to pass back and forth through the medium numerous times by means of a pair of mirrors.Additional photons are generated with each passage, leading to enormous amplification. The repeated passage also produces a beam that is highly parallel, because nonparallel radiation escapes from the sides of the medium after being reflected a few times. The four processes are pumping, spontaneous emission, stimulated emission, and absorption.
Pumping: A process by which the active species of a laser is excited by means of an electrical discharge, passage of an electrical current, or exposure to an intense radiant source. During pumping in a molecular system, several of the higher electronic and vibrational energy levels of the active species are populated. Goes from ground state to higher energy level.
Spontaneous Emission: A species in the excited state may lose energy by spontaneous emission. It is a random process, meaning the resulting photons vary in direction and phase, and the radiation is incoherent.
Stimulated Emission: The excited species is struck by a photon (same energy as the spontaneous emission photons) which cause the excited species to relax to the lower state and emit a photon of the same energy. The resulting photon is in. the same phase and direction of the photon that caused the emission thus it is a coherent radiation.
Absorption: Two photons with the exact energy of the difference between the energy states are absorbed to produce a metastable excited state.
What is needed for light amplification?
Population inversion which states that the number of photons produced by stimulated emission must equal those lost by absorption, the particles in higher state exceeds the lower state. In the inverted system, stimulated emission prevails over absorption to produce a net gain in emitted photons. Light amplification, or lasing, then occurs.
What are 3 and 4 laser systems?
3 laser system: the transition is between the excited and ground state.
4 laser system: radiation is generated by transition by a transition from highest energy level to the level that is greater than ground state. The advantage of the four-level system is that the population inversions essential for laser action are achieved more easily than in three-level systems. Therefore, the four level laser usually achieves a population inversion with a small expenditure of pumping energy.
Explain Solid State laser types.
First successful is a three level laser with a ruby crystal lasing medium. The ruby is primarily Al2O3 but contains approximately 0.05% chromium(III) distributed among the aluminum(III) lattice sites, which accounts for the red coloration and the chromium ions are the active lasing material.
A flashlamp often a low-pressure xenon lamp coiled around the cyclinder is the pumping source which creates a pulsed laser beam is produced.
Nd-YAG laser has a lasing medium of neodymium ion in a yttrium aluminium garnet crystal. It is a four level system, and has a high radiant power.
Titanium sapphire laser has a wavelength tunable in the red and near IR region. Can generate ultrashort pulses with widths in the range of tens to hundreds of femtoseconds. The lasing medium is a crystal of sapphire (Al2O3) that is doped with about 0.1% titanium. The Ti-sapphire laser is most often pumped by an argon ion laser, although the frequency-doubled.
Optical fiber has a lasing medium of an optical fiber doped with a rare earth element, such as erbium, yyterbium, or thulium. The fiber is pumped at the ends with a semiconductor diode laser. The thin fiber gives it a high surface area to volume ratio, this helps effectively dissopates generated heat more rapidly than rod lasers.
Explain Gas laser types.
Neutral atom lasers of He-Ne: cheap, reliable, low power consumption. It is in a continuous mode than pulsed mode.
Ion lasers: Active species is argon, xenon, and Kr. The argon laser has intense lines in green/blue. 4 level laser used in argon where the ions are formed by electrical or radio frequency. Argon used in fluorescence and Raman spectroscopy because of the high intensity of its lines.
Molecular lasers with carbon dioxide and nitrogen gas: Nitrogen is pumped with a high voltage spark. The excitation creates a population inversion that decays quickly resulting in short, intense pulses in UV region. Carbon dioxide used to produce monochromatic IR radiation.
Excimer laser: contains gaseous mixture of helium, fluorine, and a rare gas (argon,krypton,or xenon). Electronically excited by current followed by a fluorine reaction to create an excimer complex (ArF,KF,XeF, named that as they only stable in excited state) So since the ground state is unstable, dissociation of the complex occurs as they relax giving off a photon. Thus, population inversion if there is pumping causing high energy pulses in UV region.
Explain Dye Lasers.
Continuously tunable over a range of 20 to 50 nm in the visible region of the spectrum. By changing dyes, the wavelength range of a dye laser can be arranged to be quite broad. The laser is a four level where the active materials are organic solutions capable of fluorescing in the UV,Vis, and IR regions. However, the lower energy level is a band of energies that arise from the superposition of a large number of closely spaced vibrational and rotational energy states on the base electronic energy state. Electrons in Ey may then undergo transitions to any of these states, thus producing photons of slightly different energies.
Explain Semiconductor lasers.
Light emitting diode (LED) is a type. They are small, efficient in converting electrical to radiant, and are capable of being modulated. They can produce radiation over a broad range of wavelengths. When a voltage is applied, electrons excite into the conduction band then hole-electron pairs are created and the diode conducts. Some electrons relax and go into the valence band and the energy released is the band gap energy. LEDs are made to enchnace light production, they are made of gallium arsenic phosphide. The amount presence of arsenic and phosphide dictates the band gap energy, and thus the wavelength emission. Different emission ranges can be produced by using group III and V elements.
White light LED are composed of red,green, and blue LED into one or by illuminating phosphors with an intense blue LED.
Explain wavelength selectors.
It is needed as spectroscopic analyses require radiation that consists of limited, narrow, continuous wavelength known as bands. A narrow bandwidth enhances the sensitive of absorbance measurements and provides selectivity to emission and absorption methods. Th output of the wavelength selector would be a single wavelength radiation but instead a wavelength band occurs. Effective bandwidth is the full width as half peak heigh (FWHP) which is the inverse measure of the device quality so a narrower bandwidth is a better performance.
Explain Filters and its types.
Traditionally filters were either interference filters, which are sometimes called Fabry-Perot filters, or absorption filters. Absorption filters are restricted to the visible region of the spectrum; interference filters, on the other hand, are available for the UV, visible, and well into the IR region.
Interference filters: also known as dichroic filters as they separate light into transmitted and reflected, interferes with a narrow band of radiation. A transparent calcium/magnesium fluoride dielectric between two semitransparent metallic films. Which is then sandwiched between two plates of glass or other transparent materials. The thickness of the dielectric material is controlled to determine wavelength, a fraction passes through the metallic layer while the remainder is reflected, and the portion that passes undergoes the same thing at the second metallic film. In the second interaction, if the reflected portion is of the proper wavelength, it is then partially reflected from the inner side of the first layer in phase with the incoming light. The result is that the wavelength is reinforced due to constructive and the rest (unneeded) is out of phase so undergoes destructive interference.
Fabray perot etalon: transparent plate with highly parallel faces coated with a nonabsorbing, highly reflective material. Alternatively, an invar or quartz spacer of the chosen thickness with highly paralleled faces is placed between two mirrors to form an etalon. The bandwidth of the device is determined by the reflectivity of the coatings or mirrors, and the separation of the transmitted bands is dictated by the distance between the mirrors. The etalon may be tilted to vary the band that is transmitted. If the reflecting surfaces are configured with an air gap between them so that their separation can be adjusted mechanically, the device is called a Fabry-Perot interferometer.
Interference Wedges: consists of a pair of mirrored, partially transparent plates separated by a wedge shaped layer of dielectric material.The radiation transmitted varies continuously in wavelength from one end to the other as the thickness of the wedge varies. By choosing the proper linear position along the wedge wavelengths with a bandwidth of about 20 nm can be isolated.These filters can pass long wavelengths (long- or high-pass filters) or short wavelengths (short- or low-pass filters). By combining a high-pass and a low-pass filter, a variable bandpass filter is created.
Holo: The coherent radiation of a laser beam impinges on a beamsplitter, where it is divided into two beams, 1 and 2, shown in the figure. The two beams are redirected by the two front-surface mirrors to be recombined at the surface of the thin (10–50 μm) photosensitive film. Because the two mutually coherent beams have a fixed phase-and-intensity relationship, they produce an extremely uniform interference pattern of light and dark bars, or fringes, at the surface of the film. These interference fringes sensitize the film, which is a photographic emulsion or photoresistive polymer layer, so that the sensitized areas can be developed or dissolved away, leaving a grooved structure on the surface on the substrate. This structure is then coated with aluminum or other reflective material to produce a holographic reflection grating. volume transmission hologram: the interference fringes are formed throughout the volume of the film layer rather than just at its surface
Absorbance: have been widely used for band selection in the visible region. These filters function by absorbing selected portions of the spectrum. The most common type consists of colored glass or of a dye suspended in gelatin and sandwiched between glass plates.
What is the difference between prism and grating dispersing units?
Prisms - can be used to disperse UV, visible, and IR radiation. The
material used for their construction differs, however, depending
on the wavelength region. shorter wavelength, greater separation, has to be transparent in the range that you’re measuring
Good prism would be made out of fused silica quartz (matches cuvette)
Would have to be swapped out more
Grating - cheaper, better, disperses linearly, position of the band is linear to wavelength
Take a long time to manufacture
Explain the grating types.
Echellette: (ladder in french) has relatively broad faces from which reflection occurs and narrow unused faces. This geometry provides highly efficient diffraction of radiation, and the reason for blazing is to concentrate the radiation in a preferred direction. For the interference to be constructive, it is necessary that the path lengths differ by an integral multiple n of the wavelength l of the incident beam.
Concave: concave grating permits the design of a monochromator without auxiliary collimating and focusing mirrors or lenses because the concave surface both disperses the radiation and focuses it on the exit slit. Better for cost and increases throughput.
Holo: Holographic gratings, because of their greater perfection with respect to line shape and dimensions, provide spectra that are relatively free of stray radiation and ghosts (double images).
The quality of a monochromator depends on the purity of its radiant output, its ability to resolve adjacent wavelengths, its light-gathering power, and its spectral bandwidth.
What are polychromators and spectrographs?
Polychromator - isolates several wavelength bands
Has several exit slits
Used in atomic absorption; allows lines from several different elements to be monitored simultaneously
Spectrograph
A device w/ no exit slits, only an entrance slit
The spectrum is displayed spatially in the focal plane
The spectrum was displayed along the photographic plate with distance along the plate being related to wavelength and the density of the deposited silver being related to intensity.
What is an important characteristic of cells?
cells/cuvettes need to be transparent in the spectral region of interest
Silica used for 350 - 2000 nm
Plastic for vis
UV uses quartz or silica
Explain radiation transducers.
Converts radiant energy to electrical signal
Ideal transducer = high sensitivity, a high signal to noise ratio, a constant response over a considerable range of wavelengths
two types:
photon which absorbs radiation: the absorbed energy causes emission of electrons and the production of a photocurrent. In others, the radiation promotes electrons into conduction bands; detection here is based on the resulting enhanced conductivity (photoconduction)
shot noise limits transducer
heat
thermal noise limits transducer
Explain photon transducers.
photovoltaic cells, used in the Vis region, in which the radiant energy generates a current at the interface of a semiconductor layer -selenium- and a coppper or iron metal;When radiation of sufficient energy reaches the semiconductor, electrons and holes are formed. The electrons that have been promoted to the conduction band then migrate toward the metallic ilm and the holes toward the base on which the semiconductor is deposited. The liberated electrons are free to migrate through the external circuit to interact with these holes. The result is an electrical current of a magnitude that is proportional to the number of photons that strike the semiconductor surface. The currents produced by a photovoltaic cell are usually large enough to be measured with a microammeter.
phototubes, in which radiation causes emission of electrons from a photosensitive solid surface,consists of a semicylindrical cathode and a wire anode sealed inside an evacuated transparent envelope.The concave surface of the electrode supports a layer of photoemissive material (Section 6C-1) that tends to emit electrons when it is irradiated. When a voltage is applied across the electrodes, the emitted electrons flow to the wire anode generating a photocurrent that is generally about one tenth as great as that associated with a photovoltaic cell for a given radiant intensity. In contrast, however, amplification is easily accomplished because the phototube has a high electrical resistance
photomultiplier tubes, which contain a photoemissive surface as well as several additional surfaces that emit a cascade of electrons when struck by electrons from the photosensitive area.PMTs are limited to measuring low-power radiation because intense light causes irreversible damage to the photoelectric surface.
photoconductivity transducers in which absorption of radiation by a semiconductor produces electrons and holes, thus leading to enhanced conductivity
silicon photodiodes, in which photons cause the formation of electron-hole pairs and a current across a reverse-biased pn junction. the reversed junction creates a depletion layer that reduces the conductance of the junction to nearly zero. If radiation impinges on the chip, however, holes and electrons are formed in the depletion layer and attracted to the appropriate electrode to produce a current that is proportional to radiant power. They require only low-voltage power supplies or can be operated under zero bias. Because of this, they are widely used in portable, battery-powered instrument
charge-transfer transducers, in which the charges developed in a silicon crystal as a result of absorption of photons are collected and measured.
Explain multichannel photon transducers.
Modern multichannel transducers consist of an array of small photosensitive elements arranged either linearly or in two-dimensional pattern on a single semiconductor chip. The chip, which is usually silicon and typically has dimensions of a few millimeters on a side, also contains electronic circuitry to provide an output signal from each of the elements either sequentially or simultaneously. For spectroscopic studies, a multichannel transducer is generally placed in the focal plane of a spectrograph so that various elements of the dispersed spectrum can be transduced and measured simultaneously. Three types of multichannel devices are used in commercial spectroscopic instruments: photodiode arrays (PDAs), charge- injection devices (CIDs), and charge-coupled devices (CCDs). the individual photosensitive elements of CIDs and CCDs are usually formed as two-dimensional arrays. Charge-injection and charge-coupled transducers both function by collecting photogenerated charges in various areas of the transducer surface and then measuring the quantity of charge accumulated in a brief period. In both devices, the measurement is accomplished by transferring the charge from a collection area to a detection area.
Explain photoconductivity transducers.
The most sensitive transducers for monitoring radiation in the near-IR region (0.75 to 3 μm) are semiconductors whose resistances decrease when they absorb radiation within this range. This application of photoconductors is important in IR Fourier transform instrumentation. Crystalline semiconductors are formed from the sulfides, selenides, and stibnides of such metals as lead, cadmium, gallium, and indium. Absorption of radiation by these materials promotes some of their bound electrons into an energy state in which they are free to conduct electricity. The resulting change in conductivity can then be measured with a circuit.
What is the difference between intensity and brightness?
Intensity is the total amount of photons.
Brightness is the amount of photons in a given area
Explain the electromagnetic spectrum.
electromagnetic spectrum, the entire distribution of electromagnetic radiation according to frequency or wavelength. Although all electromagnetic waves travel at the speed of light in a vacuum, they do so at a wide range of frequencies, wavelengths, and photon energies. There is electric and magnetic vectors.
Raging martians invaded venus using X-ray guns (flip it around in terms of energy)
(radio, microwave, ir, vis, uv, x-ray, gamma)
What is percent transmission?
ratio of amount of light leaving the sample over the amount entering the sample.the greater the amount of light absorbed by the sample, the smaller the percent transmittance. tells you how much of the entering light is exiting the sample
Explain dispersion, diffraction, reflection, and refraction.
dispersion: The separation of visible light into its different colors, The variation in refractive index of a substance with wavelength or frequency is called its dispersion
diffraction: Diffraction is the spreading out of waves as they pass through a medium.
reflection: light bounce off of an object, When radiation crosses an interface between media that differ in refractive index, reflection always occurs. The fraction of reflected radiation becomes greater with increasing differences in refractive index.
refraction: Refraction is the bending of light (it also happens with sound, water and other waves) as it passes from one transparent substance into another. the density of the material matters because if it is more dense then itll slow down causing the bend (towards normal)
Explain GC source.
A regulated and purified carrier gas source, which moves the sample through the GC.The carrier gas must be pure. Contaminants may react with the sample or the column, create spurious peaks, load the detector and raise baselines, and so on. A high-purity gas with traps for water, hydrocarbons and oxygen is recommended.
Explain how separation occurs in the GC, with all components.
Gas chromatography (GC) is a laboratory technique that separates mixtures into individual components. It is used to identify components and to measure their concentrations.GC creates a time separation. It does this by passing the vaporized mixture (or a gas) through a tube containing a material that retards some components more than others. This separates thc components in time. After detection, the result is a chromatogram, where each peak represents a different component of the original mixture. The appearance time can be used to identify each component; the peak size (height or area) is a measure of the amount. A regulated and purified carrier gas source, which moves the sample through the GC:
• An inlet, which also acts as a vaporizer for liquid samples
• A column, in which the time separation occurs, placed in a well-controlled oven as separations are highly temperature-dependent,
• A detector, which responds to the components as they occur by changing its electrical output
• Data interpretation of some sort
When a vaporized component is presented with a gas phase and a coating phase, it divides between the two phases according to its relative attraction to the two phases.
• The “attraction” can be solubility, volatility, polarity, specific chemical interaction, or any other property that differs from one component to another.
• If one phase is stationary (the coating) and the other is moving (the carrier gas), the component will travel at a speed less than that of the moving phase. How much less depends on the strength of the attraction.
• If different components have different “attractions”, they will separate in time.
Explain GC detector.
FID:An air/hydrogen flame creates very few ionized particles. However, if a carbon-containing material enters the flame, ion production increases.
The carrier gas from the column mixes with hydrogen and is burned in air. The FID uses two electrodes, one of which is often the jet where the flame burns, and a polarizing voltage to collect the ions from the flame. When a component appears, the collected current rises. After amplification, the current creates the chromatogram. The FID responds to anything that creates ions in a flame, which is essentially all organic compounds
Why doesn’t the photomultiplier work in IR?
IR radiation does not have enough energy to cause the initial cascade of electrons.
What are the two characteristics of light?
Light acts as both a wave and a particle, but are not mutually exclusive (not 1 or the other)
They are complimentary
Explain Snells law.
Snell’s law, in optics, a relationship between the path taken by a ray of light in crossing the boundary or surface of separation between two contacting substances and the refractive index of each. Thus, the path of a light ray is bent toward the normal when the ray enters a substance with an index of refraction higher than the one from which it emerges; and because the path of a ray of light is reversible, the ray is bent away from the normal when entering a substance of lower refractive index.
what is the difference between spectroscopy and spectrometry?
Spectroscopy is the science of the radiation and matter interactions and spectrometry is the measurement of intensity
Explain photons.
viewed as a stream of discrete particles, or wave packets, of electromagnetic energy called photons. The energy of a photon is proportional to the frequency of the radiation.
What is plane polarized?
all oscillations of either the electric or the magnetic field lie in a single plane
Explain amplitude, frequency, period, wavenumber, and wavelength.
amplitude A of the sinusoidal wave is shown as the length of the electric vector at a maximum in the wave
frequency n is the number of oscillations of the field that occur per second1 and is equal to 1/p. frequency of a beam of radiation is determined by the source and remains invariant
The time in seconds required for the passage of successive maxima or minima through a fixed point in space is called the period p of the radiation
wavenumber is the amount of wavelengths in a given distance, it is a useful unit because, in contrast to wavelength, it is directly proportional to the frequency, and thus the energy, of radiation
wavelength is the linear distance between any two equivalent points on successive waves (e.g., successive maxima or minima)
What is refractive index?
The refractive index of a medium is one measure of its interaction with radiation found by dividing velocity in a vacuum by velocity in a medium. Since the velocity of radiation in matter is wavelength dependent and since velocity in a vacuum is independent of wavelength, the refractive index of a substance must also change with wavelength