Core practical 4 - investigate the hydrolysis of halogenoalkanes
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Write an equation for the reaction of 1-bromobutane with water.
CH3CH2CH2CH2Br + H2O → CH3CH2CH2CH2OH + H+ + Br-
In these reactions a precipitate forms. Identify the precipitate formed when the halogenoalkane is 1-iodobutane.
Silver iodide
Explain why ethanol is used in these reactions
The halogenoalkanes are insoluble in water. Using ethanol ensures that the halogenoalkane dissolves so it can react with the water molecules.
Explain why water is able to act as a nucleophile.
Water has lone pair(s) of electrons on the oxygen atom
Explain why water is used as the nucleophile rather than hydroxide ions?
If hydroxide ions were used, a precipitate of silver hydroxide would form instantly.
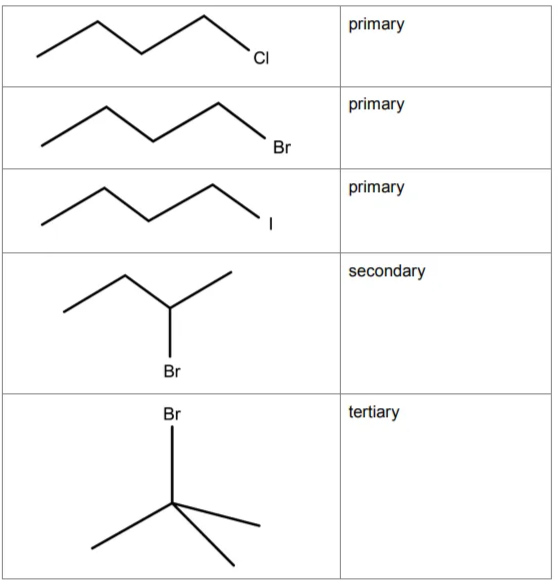
Draw skeletal formulae for each of the halogenoalkanes used in this investigation (there are 5 of them). Classify each halogenoalkane as primary, secondary or tertiary.