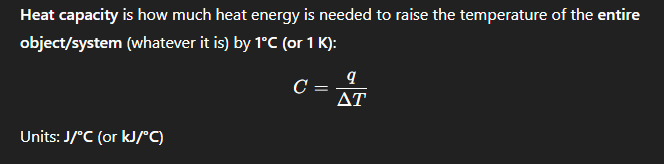General Chem 2: Unit 1 (Thermodynamics)
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
28 Terms
1st law of Thermodynamics
Energy cannot be created nor destroyed only transferred
All forms of energy are either ___ or ___ energy
kinetic or potential
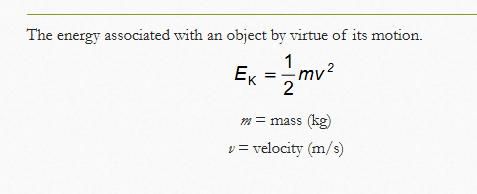
What is the formula to determine kinetic energy and what are the units?
mass = kg, v = m/s, and the answer will be in joules


A state function is best described as one that depends on the ___ and ___ points of a process, whereas a path function depends on the ___ taken.
starting, end, route
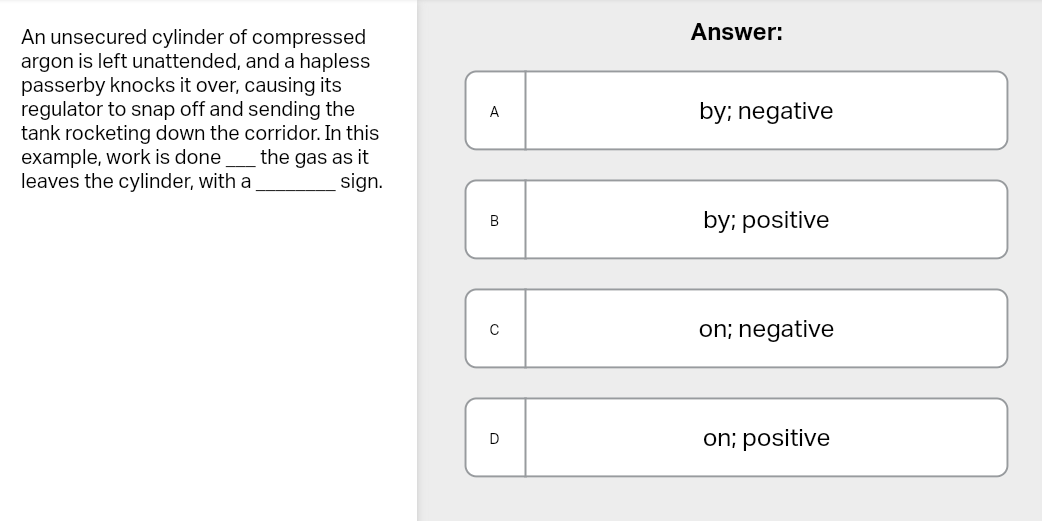
Quick memory trick
Compression = +w
Expansion = −w
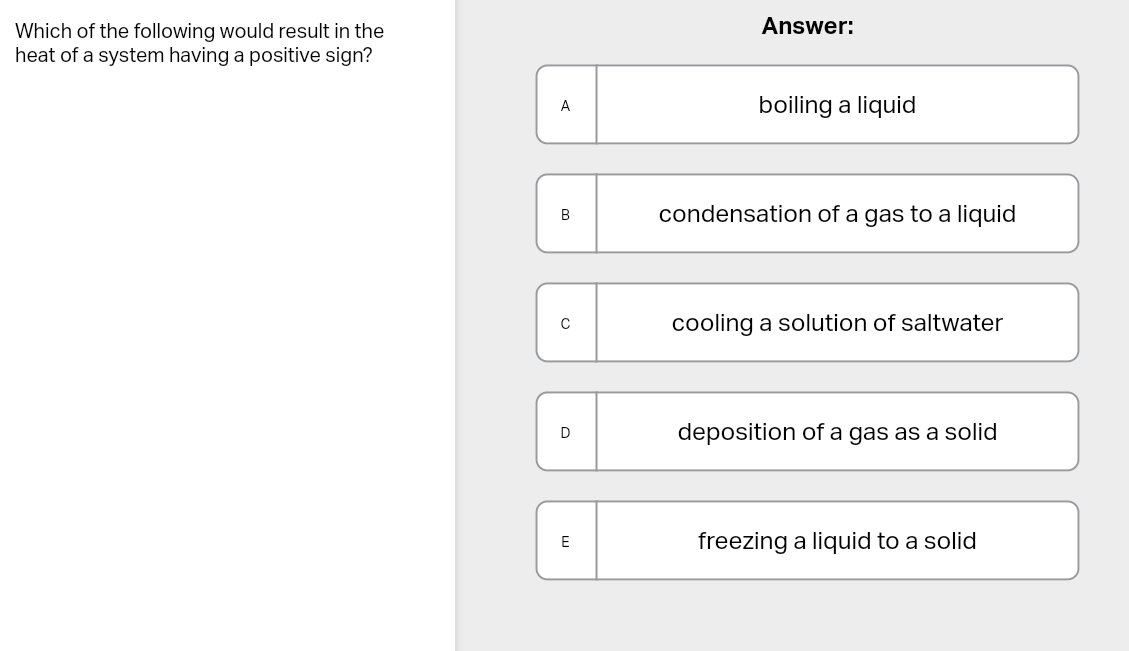
A: because it’s referring to absorbing heat.

D:
If you push or pull and it actually moves (in the direction of the force), you did work.
Push a box and it slides → work
Hold a heavy box still → no work (because no movement)
Exothermic means there is a transfer of heat from the ___ to the ___. It ___ the heat as a product
system, surroundings, releases
Endothermic is the transfer of heat from the ___ to the ___. Heat is the part of the ___
surroundings, system, reactants
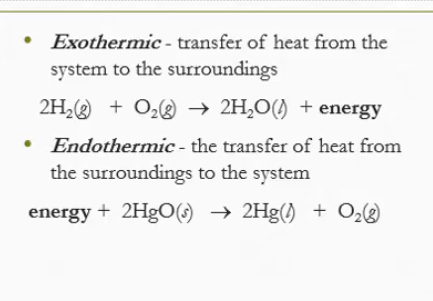

A) Exothermic your hand is releasing heat
B) Endothermic the ice is absorbing heat
C) Endothermic water is absorbing heat
D) Exothermic vapor is releasing heat
E) Endothermic ice cream is absorbing heat
Open systems can exchange ___ and ___ with the surroundings, like boiling water.
matter, heat
Closed systems can exchange only ___. Example would be a sealed bottle of water that you heat.
energy
Isolated systems exchange neither ___ nor ___. Example is an ideal thermos although real ones aren’t perfect.
energy, matter
Heat capacity is how many energy it takes to raise the temperature of an ___ ___ by 1 degree Celsius/Kelvin, whereas specific heat capacity is how much heat it takes to raise the temperature of ___ ___ of a substance by 1 degree Celsius/Kelvin.
entire sample, one gram
An adiabatic process means the net energy transferred is ___
zero
Isothermal process regards the total temperature change remains ___
constant
State function refers only to the ___ and ___ values and does not care about the ___ taken. For example, ___ in the heat formula is an example of a state function.
final, initial, (delta) T
Path function refers to the ___ taken as there may be multiple methods to get to the ___ result. For instance, the heat formula is considered a path function
route, final,
There is a transfer of energy between a system and its surroundings until ___ is reached.
equilibrium
If the heat formula q > 0, that means the reaction was ___
endothermic (absorbed heat)
If the heat formula is q < 0, that means the reaction was ___
exothermic (released heat)
When work is greater than 0, that means work is done through ___ (on the system), whereas if it is less than 0, that means the work was is done through ___ (by the system)
compression, expansion

both negative because heat is released and work is done on the surroundings


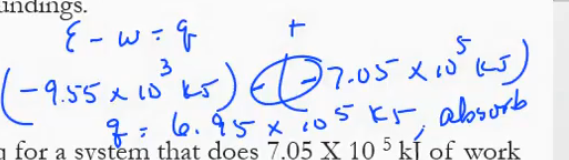
A calorimeter is a device to measure the ___ transferred
heat
What is the formula for heat capacity, and what are the units?