E1 Structure of the Atom
E1.1 Rutherford’s gold foil
Studies alpha particle scattering. Beam of high E α particles fired at gold foil and detector on other side: nr of part + angle of refraction of α particles.
Some were
(1) reflected at >90o ( small nr of part) - sug. small dense nucl
(2) transmitted straight line (majority) -suggest lotof empty space
(3) refracted at angle (θ <10o) -suggest + central nucleus
This suggests: atoms consist of small, dense, + charge nuclei surrounded by negatively charged electron cloud.
Atom diameter: 10-10m, nucleus diameter: 10-15 m
E1.2 Nuclear Notation
All matter is made of atoms: proton + neutron + electron
can measure charge and mass in C/e/kg/u
Nucleus is described by: AZX notation (A=nucleon nr)(Z=prot nr)(X=chemical symbol of element)
A = Prot + neutr
Z = Prot (determines X)
1/13
Earn XP
Description and Tags
atom structure
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
E1.1 Rutherford’s gold foil
Studies alpha particle scattering. Beam of high E α particles fired at gold foil and detector on other side: nr of part + angle of refraction of α particles.
Some were
(1) reflected at >90o ( small nr of part) - sug. small dense nucl
(2) transmitted straight line (majority) -suggest lotof empty space
(3) refracted at angle (θ <10o) -suggest + central nucleus
This suggests: atoms consist of small, dense, + charge nuclei surrounded by negatively charged electron cloud.
Atom diameter: 10-10m, nucleus diameter: 10-15 m
E1.2 Nuclear Notation
All matter is made of atoms: proton + neutron + electron
can measure charge and mass in C/e/kg/u
Nucleus is described by: AZX notation (A=nucleon nr)(Z=prot nr)(X=chemical symbol of element)
A = Prot + neutr
Z = Prot (determines X)
E1.3 Atomic spectrum
When atoms emit/absorb light of certain wavelengths; emission and absorption. These provide evidence that electrons in atoms can only transition between discrete atomic energy levels.
Each element has its own spectra, can be used as a fingerprint.
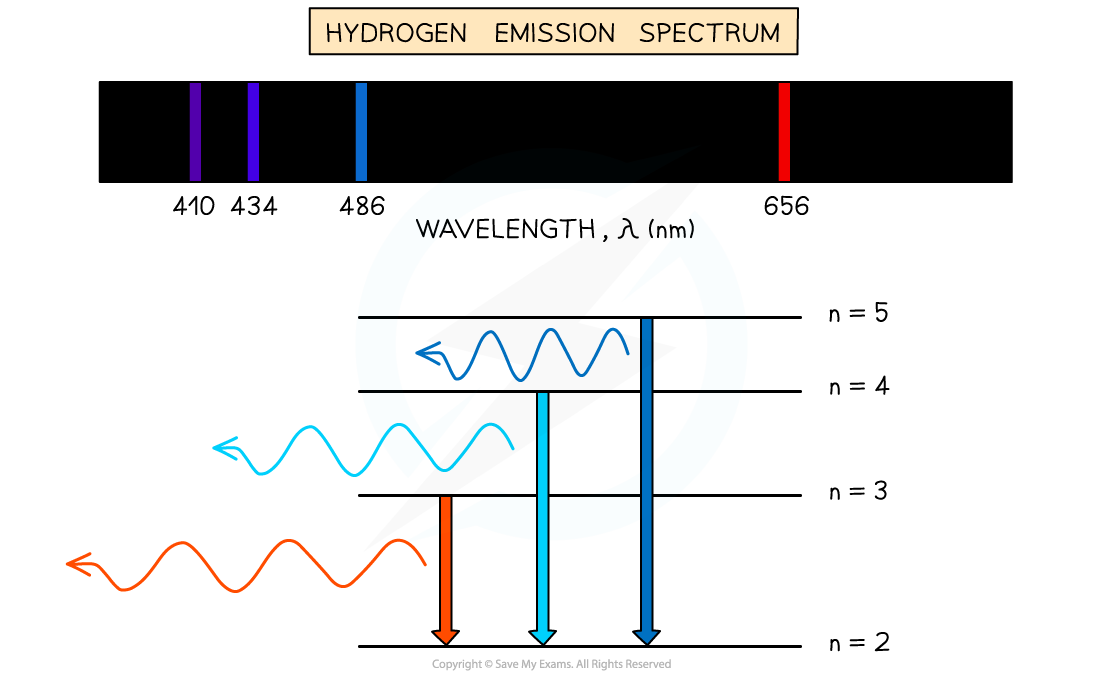
E1.4 Emission spectra
Produce by heating low-pressure gas. Excite e- to higher E level
When they transition back to lower E level; emit photon
Each transition corresponds to specific wavelength of light → observable spectral line
Results in emission spectrium containing set of discrete wavelengths represented by coloured lines on black background
Eg. Hydrogen:
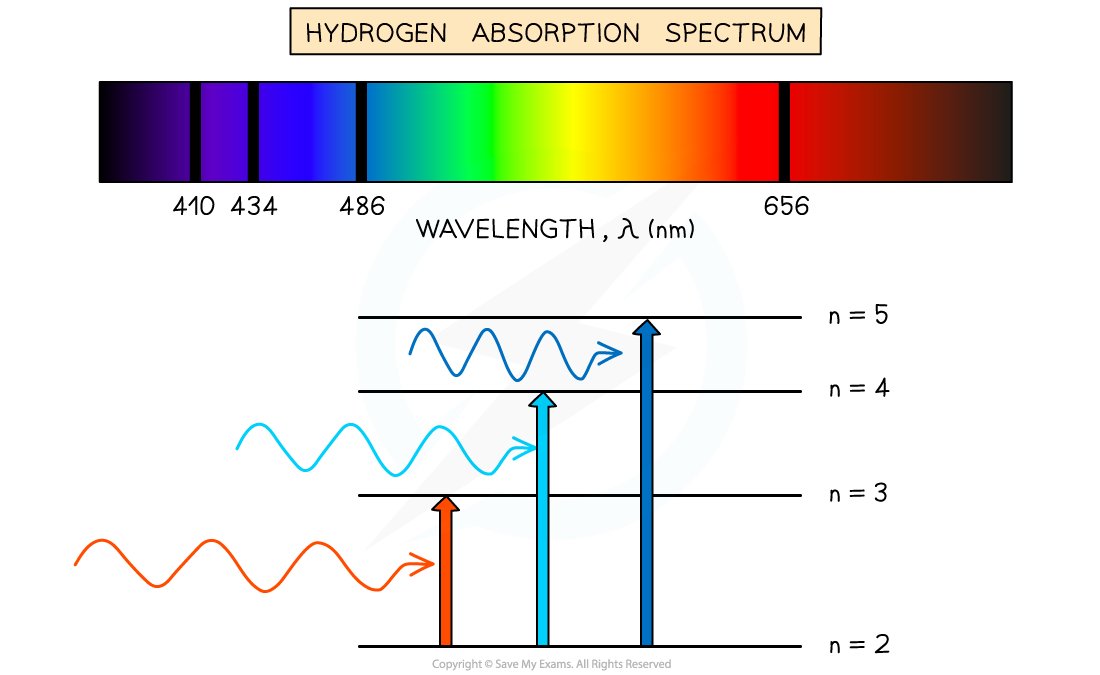
E1.5 Absorption Spectra
Cool, low pressure gas: only photons with exact E required to excite e- will be absorbed.
Each absorbed photon → specific wavelength → dark line on continuous spectrum
Results in absorption spectrium containing discrete wavelengths represented by dark lines on coloured background → opposite of emission spectrum
E1.6 Photon Energy
Photon: massless packet/quantum of EM energy. → discrete/specific packet of energy, all transferred in 1 go.
E1.7 Atomic energy level
Electrons in atom occupy energy states → levels. Lowest possible energy level; most stable configuration. When e- absorb/emit photon, moves between E levels or leaves atom completely.
E1.8 Exciting/Ionizing an electron + Energy photon
Excitation: when e- moves up an E level; absorb photon, de-excite emits photon of exact same energy levels
Ionisation: when e- is removed from atom.
Ionization energy: minimum energy required to remove an electron from ground state of atom
The energy of the emitted photon is equal to the difference in energy between the energy levels in the transition (high → low).
Energy of a photon E = hf = hc/λ (h = 6.63 × 10-34 Js)
If you look at diagram: up is absorbed, down is emitted (same E)
E1.9 Nuclear radius
Radius of nucleus depends on nucleon nr (prot+neutr) → experiment →
R = RoA1/3 (R0 = 1.2×10-15 m = Fermi radius; Hydrogen nucleus w 1 proton) (A = nucleon/mass nr = prot+neutr)
E1.10 Nuclear Density
V = 4/3 πR3 = 4/3 πR03A
m = Au (Nucleon nr, u=atomicmassunit (1.661×10-27kg)
ρ = m/V = nuclear density = u/(4/3 πR03)
E1.12 Deviations of Rutherford scattering
He predicted that as scattering increases, nr of alpha particles at that angle decreases. However, at high energies of the alpha particles, this deviated → no back scattering → go to 0.
Rutherford assumed pure electrostatic repulsion, when in reality, at high E, they interact with nucleus via strong nuclear (force holding nucleons tg).
Therefore, Rutherford is also evidence for strong nuclear, by pushing alphas away
With smaller target nuclei (non gold) and higher energy alpha → more scattering as less effected by electric repulsion.
E1.13 Bohr Model (of Hydrogen)
Line spectra are drawn as to show different energy levels. electrons are able to jump/transition between these specific energy levels. These transitions are categorised into series;
Ground State: n=1 Lyman series (low wavelength → increas w n
E-levels ; Balmer → Ritz-Pashen → Brackett → Pfund
To n=2 produces visible light.
Bohr model of Hydrogen: e- move in fixed orbits, restricted to certain radius.
Discrete energy of transitions in Bohr Model → E = -13.6/n2 eV
E = photon energy, n = 1,2,3,4,5…. GIVE THIS IN…eV
E1.14 Bohr condition
nh/2π = mvr (v = v of e-) (m=mass of e-)(r=rad of orbit)(n=E-level)