Membrane structure and composition, and Osmotic Balance (2.1 - 2.2)
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What is the function of the plasma membrane?
The plasma membrane separates the intracellular fluid (ICF) from the extracellular fluid (ECF), maintaining the internal environment of the cell and regulating substance exchange.
What is the structure of the plasma membrane?
The membrane is a lipid bilayer composed primarily of amphiphilic phospholipids. The hydrophilic (water-attracting) ends face outward, while the hydrophobic (water-repelling) ends face inward, forming a two-molecule-thick layer.
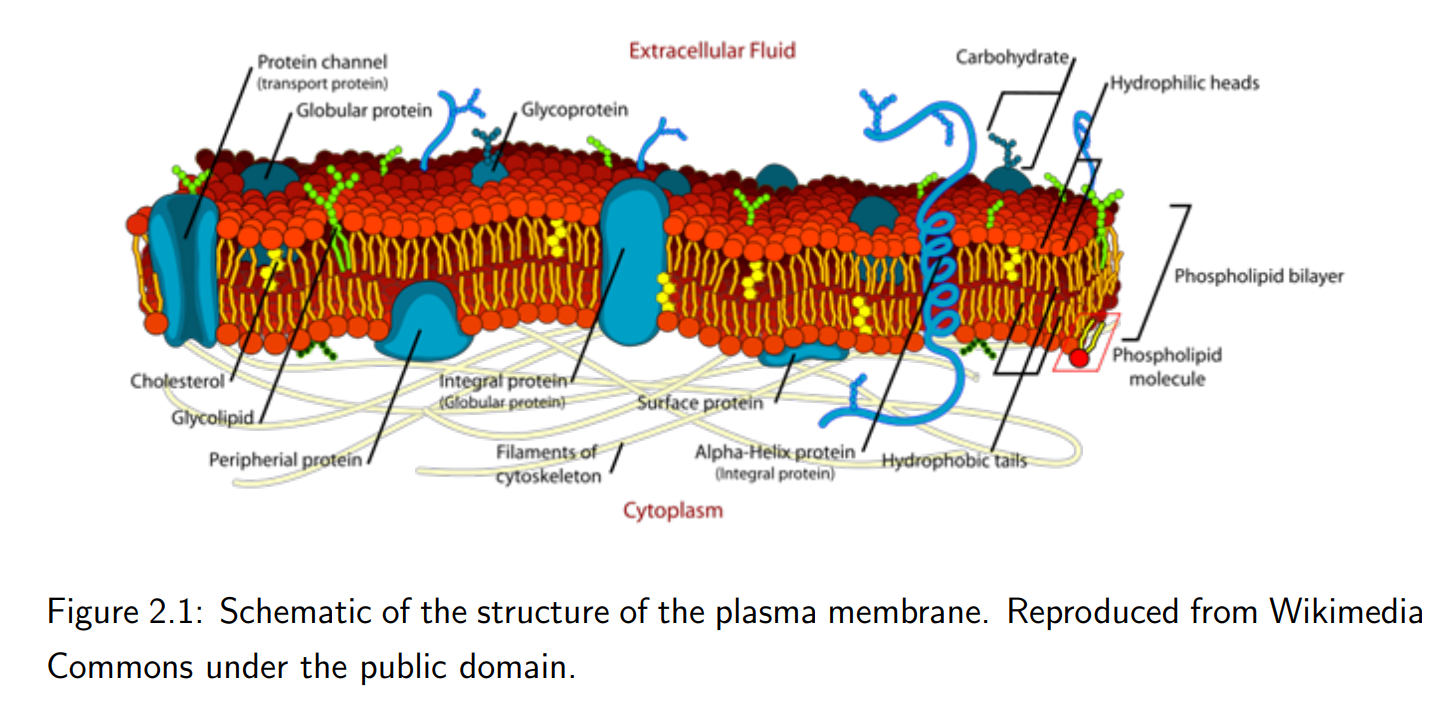
What role do proteins play in the plasma membrane?
Membrane proteins are embedded in the lipid bilayer, facilitating functions such as transport (e.g., channels and pores), signaling, and structural support.
What ions are dominant inside and outside the cell?
- Inside the cell (ICF): High concentrations of K^+ and negatively charged molecules like proteins.
- Outside the cell (ECF): High concentrations of Na^+ and Cl^-.
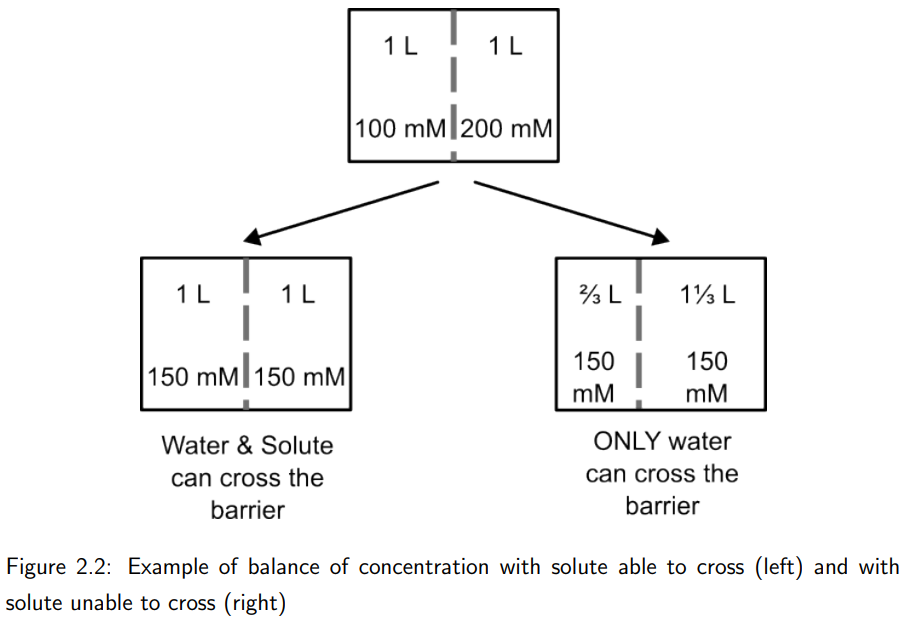
What happens when the barrier between two solutions only allows water to cross?
Water moves to balance solute concentrations, resulting in unequal volumes. For example, if one solution is 100 mM and the other is 200 mM, water will flow into the higher concentration side until equilibrium is reached.
This could be described as “balancing water concentrations”.
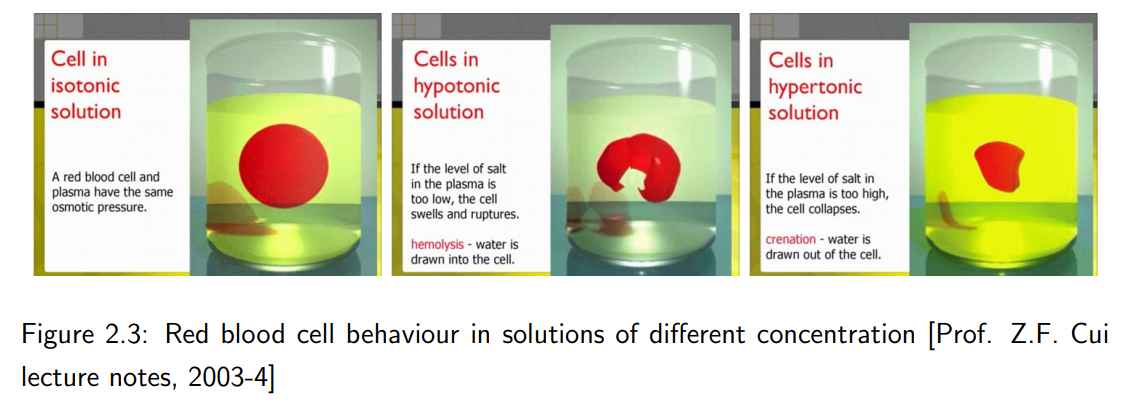
What are isotonic, hypotonic, and hypertonic solutions?
1. Isotonic solution: The external solute concentration is equal to the internal, and cell volume remains constant.
2. Hypotonic solution: The external solute concentration is lower, causing water to enter the cell, and the cell swells.
3. Hypertonic solution: The external solute concentration is higher, causing water to leave the cell, and the cell shrinks.
What happens when solute Q is permeable to the membrane but not P?
If Q can cross the membrane:
1. The concentration of Q must equilibrate, being the same inside and outside the cell.
2. The total solute concentration must also equalize between the intracellular and extracellular environments.
Outcome: These conditions can only be satisfied if the concentration of impermeable solute P is zero. This causes water to continue entering the cell, leading to unregulated expansion, potentially resulting in the cell expanding to infinite size.
What are the two equilibrium conditions explored so far?
Water concentration (or total solute concentration) must balance across the membrane (total concentration inside must equal outside).
Individual permeable solute concentrations must balance across the membrane.
What is osmotic balance?
Osmotic balance refers to the regulation of water movement between the ICF and ECF to maintain equal osmotic pressure, which prevents cell swelling or shrinking.
What is osmolarity, and how is it calculated?
Osmolarity measures the total solute concentration in a solution, accounting for dissociation of solutes. For example:
- A 0.1 M glucose solution has an osmolarity of 0.1 Osm.
- A 0.1 M NaCl solution has an osmolarity of 0.2 Osm (since NaCl dissociates into Na^+ and Cl^-).
What is the relationship between osmotic pressure and osmolarity?
Osmotic pressure (\pi) is given by van ’t Hoff’s law:
\pi = RT (\phi i c)
where R is the gas constant, T is temperature, \phi is the osmotic coefficient, i is the number of ions formed per solute molecule, and c is the solute concentration.
How is osmolarity calculated, and what are examples?
Definition: Osmolarity is the total solute concentration in a solution, accounting for dissociation of molecules.
Examples:
1. A solution with 0.1 M glucose and 0.1 M urea has an osmolarity of 0.2 Osm (both molecules do not dissociate).
2. A 0.1 M solution of NaCl has an osmolarity of 0.2 Osm, as NaCl dissociates into Na^+ and Cl^-.
Note: The osmolarity may be slightly lower in practice if ions interact in solution, though this is uncommon in biological systems.
What are the two equilibrium conditions explored so far (version 2)?
Osmotic equilibrium (total solute concentration): The osmotic pressures inside and outside the cell must balance to prevent water movement across the membrane.
Concentration equilibrium: Permeable solutes, must have equal concentrations on both sides of the membrane to avoid net diffusion.
(replaced condition 1 with “osmotic equilibrium” as it takes ion disassociation into account)