Unit 4 Chemistry
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Covalent bond
Electrons simultaneously attracted by more than one nucleus
Coordinate covalent bond
When the nucleus of one atom attracts 2 electrons while the nucleus of the other attracts none
Bond energy
the average amount of energy needed to break one mole of that particular covalent bond.
Lattice energy
amount of energy released when 1 mole of ionic bonds form
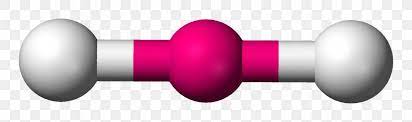
Name geometry and angle(s)
Linear; 180
Name geometry and angle(s)
Trigonal Planar; 120
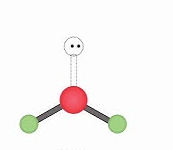
Name geometry and angle(s)
Bent; <120
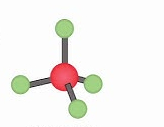
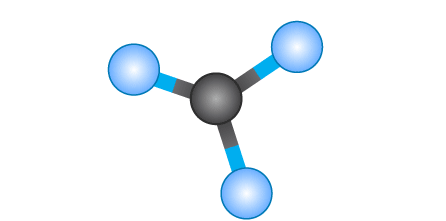
Name geometry and angle(s)
Tetrahedral; 109.5
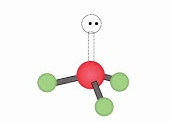
Name geometry and angle(s)
Trigonal Pyramid; <109.5
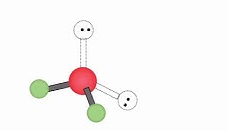
Name geometry and angle(s)
Bent; <109.5
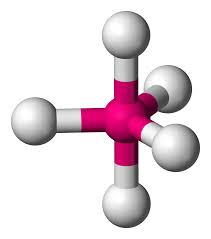
Name geometry and angle(s)
Trigonal bipyramidal; 90, 120
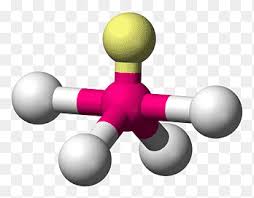
Name geometry and angle(s)
See-saw; <120, <90
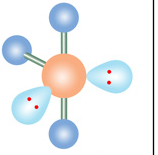
Name geometry and angle(s)
T-structure; <90
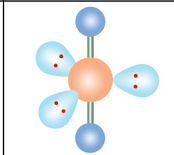
Name geometry and angle(s)
Linear; 180
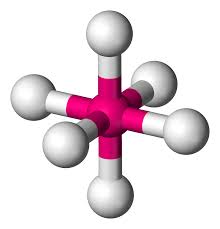
Name geometry and angle(s)
Octahedral; 90, 90
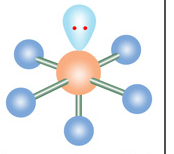
Name geometry and angle(s)
Square pyramidal; 90, <90
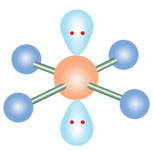
Name geometry and angle(s)
Square planar; 90
Which elements are not distorted by hydrogen?
Hydrogen does not distort the atomic orbitals of period 3-7 central atoms, so it doesn't affect the geometry.
Only non-metals in periods _______ can form “extra-octet” structures
3-7
Why can’t period 2 non-metal central atoms form bonds with 5-6 atoms?
They are too small so, they would be too close to terminal atoms and there would be too much repulsions.
Which atoms can form a sub-octet?
Al and B form sextet
Be forms only 2 bonds
Electronegativity
The ability for an atom to attract covalently bonded electrons to itself
Explain bond length.
The greater the bonding e-density (or character/order), the shorter the bond length and the greater the bond strength AND higher the bond energy
Define resonance
when “pi” electrons are shared amongst 3 or more atoms
What are delocalized electrons
the pi electrons that are shared among 3+ atoms
Molecules will exhibit resonance if…
more than one valid Lewis structure can be drawn for the molecule
Visible light spectrometer
Measures the different wavelengths of visible light emitted by a source
What does infrared spectroscopy help find?
The kinds of covalent bonds in an unknown molecule
How does an IR spectrometer work?
It will emit IR radiation to a given molecule which will cause different vibrations for different covalent bonds.
Microwave spectroscopy
Helps determine molecular geometry as well as polarity of a molecule.
How to read mass spectrometry?
Determines the abundance of a certain compound and their fragments
How to read photoelectron spectrometry?
determines the energy needed to eject a given electrons from a source using the photoelectric effect (while all the other electrons are still there)