Metabolism / Excretion
1/119
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
120 Terms
What is Biotransformation of a drug
Drug Metabolism - The process whereby drugs in the body undergo transformations catalyzed by Enzymes
Drug Elimination
The irreversible loss of drug from the body
3 Main routes of Drug Elimination
Kidney
Hepatobiliary System
Lungs
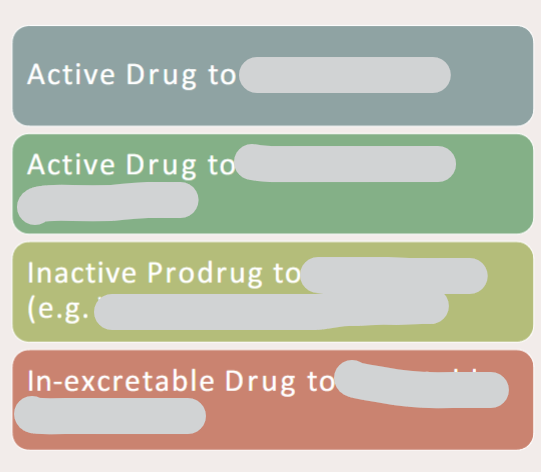
In drug metabolism how can drugs be changed

What is First-Pass Metabolism
• a.k.a. Presystemic Metabolism
• Drug metabolised (Concentration Decreased) before it reaches Systemic Circulation and, ultimately, target
• The drug is absorbed from the GI tract and passes via the portal vein into the liver where some drugs are metabolised.
First pass metabolism can occur in what organs
the gut, liver and the lung
What would be broken down by first pass metabolism in the gut (2 examples)
benzylpenicillin
insulin
What would be broken down by first pass metabolism in the liver (2 examples)
Lignocaine
Glyceryl Trinitrate (GTN)
What alternative routes of administration can be used to avoid the first pass effect
Alternative routes of administration = intravenous, intramuscular & sublingual avoid the first-pass effect
How does metabolism in the liver work? What structures & enzymes?
Enzymes: Hepatic Microsomal Enzyme System
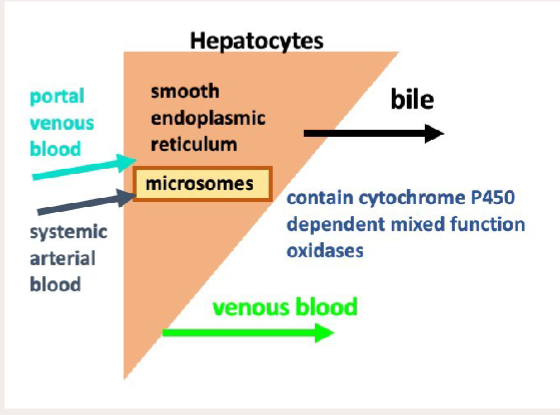
There are 2 main phases of drug metabolism. What is the purpose of both

There are 2 main phases of drug metabolism. What is the process of both

What reactions occur in phase 1 of metabolism
• Oxidation
• Reduction
• Hydrolysis
(functionalisation reactions)
What enzymes are responsible for phase 1 metabolism of drugs
Cytochrome P450 (CYP450) enzymes
Most utilised forms of Cytochrome P450 (CYP450) for phase 1 metabolism
• CYP3A4
• CYP2D6
Function of Cytochrome P450 (CYP450) enzymes in phase 1 metabolism
Converts Non-Polar Drugs to Polar Drugs (i.e. lipophilic → hydrophillic)
CYP1 function
Primarily metabolize carcinogens, some drugs as well
CYP2 function
Metabolize many important drugs
CYP3 function
Most abundant family in human liver, metabolize many important drugs (60%)
Name 4 Factors affecting CYP450 Activity

Polymorphisms most often occur in what form of CYP450 enzymes
Most commonly occur with CYP2D6
Consequences of polymorphisms of CYP450 enzymes
Alter rate of metabolism - Can Increase or Decrease rate of Metabolism
Function of enzyme inducers
Cause Enzyme to Work Faster
What 2 ways can enzyme inhibitors effect enzymes
• Inhibit action of the Enzyme
• Propagate Drug Effect
Name 7 enzyme inducers
SCRAP GP

Name 12 Enzyme Inhibitors
SICKFACES.COM
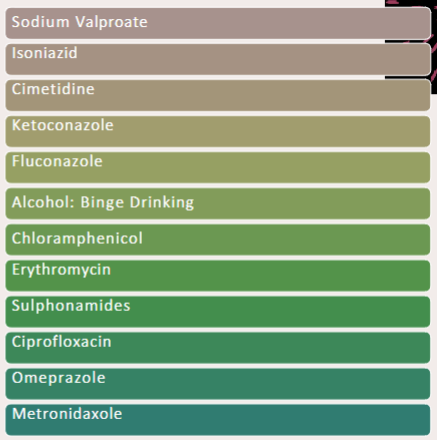
Warfarin is metabolised by what
Metabolised by CYP450 (CYP1A2 / CYP3A4) (1, 2, 3, 4)
What effect would ciprofloxacin have on warfarin action
Raise warfarin levels - increase anticoagulant ability
What happens to CYP450 enzyme activity in liver dysfunction?
CYP450 enzyme efficacy decreases, leading to slower drug metabolism.
How does reduced CYP450 function affect drug levels?
It causes increased plasma drug concentrations and a higher risk of toxicity.
How does age affect enzyme function
Neonates / Elderly have Decreased Enzyme Function
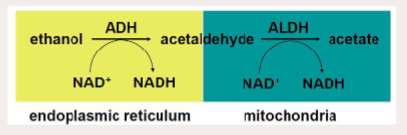
Steps of ethanol metabolism & enzymes involved & location
Two Oxidative Steps involved:
• Dehydrogenation (Oxidation) from Alcohol to Acetaldehyde (Alcohol Dehydrogenase)
• Second Oxidative Step: Acetaldehyde → Acetate (Aldehyde Dehydrogenase)
• Acetate then released to blood for subsequent oxidation to CO2 by other tissues
Disulfiram / Antbuse use
Alcohol Cessation
Disulfiram / Antbuse is soluble in what (lipid/water)
Highly lipid soluble
Disulfiram / Antbuse bioavailability %
80%
Disulfiram / Antbuse accumulates where
in tissue
Disulfiram / Antbuse mechanism of action
Irreversible inhibits Oxidation of Acetaldehyde to Acetate
Competes with CoFactor: Nicotinamide Adenine Dinucleotide (NAD) for binding sitse on ADLH
Disulfiram / Antbuse consequence of MOA
Prevents oxidation of Acetaldehyde
Propagates the unpleasant side effects of Acetaldehyde
What can occur as a result of an aldehyde dehydrogenase mutation
Metabolise Ethanol more quickly
or
Metabolise Acetaldehyde less efficiently
Accumulation of Acetaldehyde → Vasodilation → Facial Erythema
Give 5 examples of phase II conjugation reactions
Methylation (Methyl Transferases)
• Acetylation (Acetyl Transferases)
• Sulfation
• Glutathione Conjugation
• Glucuronidation
Fill in this for phase II reactions
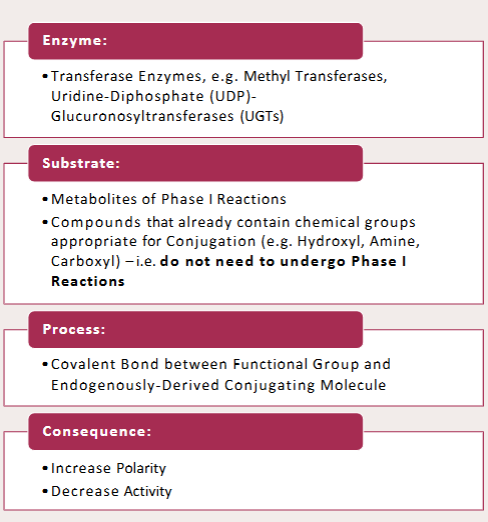
Glucuronidation is one of the phase II Conjugation Reactions. Name 3 drugs associated with it
Acetaminophen (Paracetamol), Morphine, Oxazepam
Glucuronidation is one of the phase II Conjugation Reactions. Name the enzyme associated with it
UDP-Glucuronyl Transferases
Glucuronidation is one of the phase II Conjugation Reactions. Name the substrate associated with it
Uridine Diphophate
Glucuronidation is one of the phase II Conjugation Reactions. Name the product associated with it
Uridine 5’-Diphosphate
(Catalyses the transfer of Glucuronic acid from the cofactor UDP-G A to a substrate to form β-d-glucopyranosiduronic acids (glucuronides), metabolites that are sensitive to cleavage by β- glucuronidase)
Prodrug
inactive compounds that are metabolised into their active, therapeutic forms
Tamoxifen is the prodrug of ______ which is _____x more active than it
4-hydroxytamoxifen
30-40x
Morphine (CYP2D6) prodrug
Codeine → Morphine (CYP2D6)
enalprilat prodrug
Enalapril → enalprilat
prednisolone prodrug
Prednisone → prednisolone
5-ASA prodrug
Sulfasalazine → 5-ASA (5-aminosalicylic acid)
The conversion of Sulfasalazine → 5-ASA has what effect on its action
It allows it to have site specific action
Give an example of a drug that when metabolised can produce toxic metabolites
Paracetamol (acetaminophen)
Use for Sulphasalazine
treat inflammatory conditions of the gut and joints
What percentage of the Active Metabolite: Sulphasalazine reaches the colon
Approx 90%
What happens the Active Metabolite: Sulphasalazine when it reaches the colon
(metabolised by? produces?)
Metabolised by GI Bacteria
Product: Sulfapyridine and Mesalazine
T/F: Sulphasalazine exerts it’s therapeutic effect locally
True
Codeine (3-methoxymorphine) undergoes what process by CYP2D6 to produce morphine
Codeine (3-methoxymorphine) undergoes demethylation by CYP2D6 to produce morphine
codeine has what % the analgesic potency of morphine
20%
What % of the population have codeine resistance
10%
What causes codeine resistance
absence of Demethylating Enzyme that converts it to Morphine
Codeine is often given in combination with what
Often combined with paracetamol or non-steroidal anti-inflammatory drugs (NSAIDs) in proprietary analgesic preparations
(• Synergistic effect
• Potential for safety concerns)
Name 2 phase II reactions that break down paracetamol
(enzyme & product)
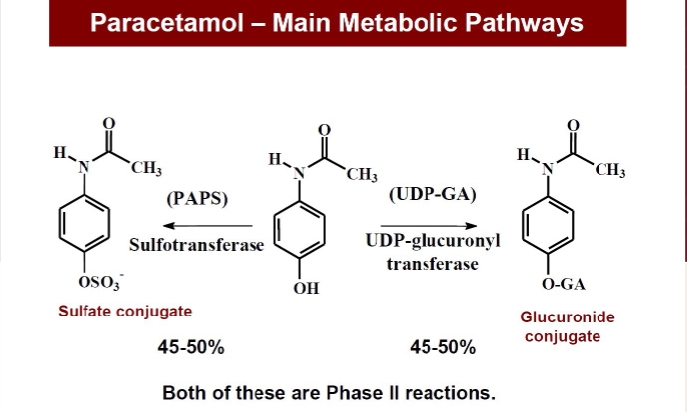
Explain the minor pathway that leads to a toxic metabolite of paracetamol (& name that toxic metabolite)
Paracetamol —(P450 system)→ NAPQI (toxic) —(Glutathione transferase)→ Glutathione conjugate
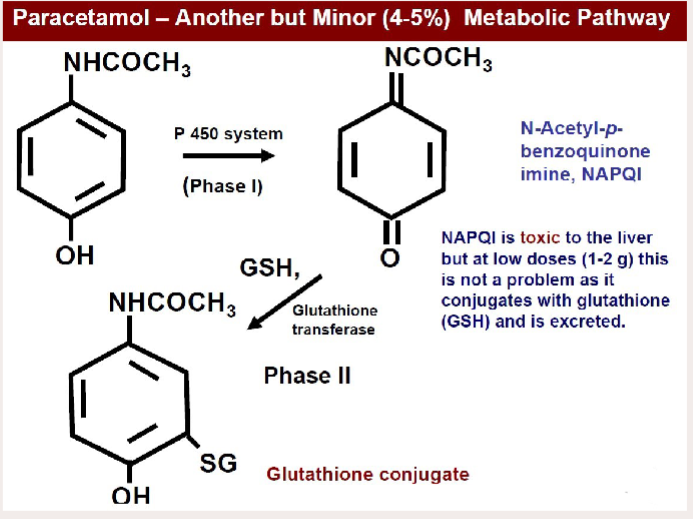
how does a paracetamol overdose occur
Glutathione pathway becomes saturated

Effect of habitual & binge drinking of alcohol on these P450 enzymes: CYP1A2, CYP2E1 and CYP3A4
Habitual Use: Induces Enzymes (up to 4x the level of non-drinkers)
Binge: Inhibits Enzymes
How does the habitual use of alcohol affect paracetamol metabolism
When CYP2E1 and CYP3A4 are induced by alcohol, a greater amount of paracetamol is converted to NAPQI.
CYP1A2, CYP2E1 and CYP3A4 are the p-450 isoforms which Oxidise paracetamol to NAPQI.
Hence in alcohol abusers, toxicity can occur with only slightly greater than recommended doses of paracetamol
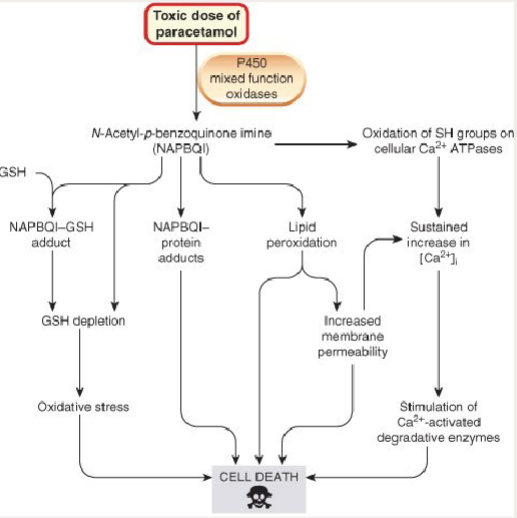
Paracetamol OD Antidote
N-Acetylcysteine (NAC)
N-Acetylcysteine (NAC) is a precursor to what
Precursor to L-Glutathione
N-Acetylcysteine (NAC) works as a _______ donor
Behaves as Glutathione Donor
(increases Phase II of paracetamol metabolsim)
How does N-Acetylcysteine (NAC) work as a Paracetamol OD Antidote
Minimises Oxidative Stress
Reduces risk of Fulminant Hepatic Failure
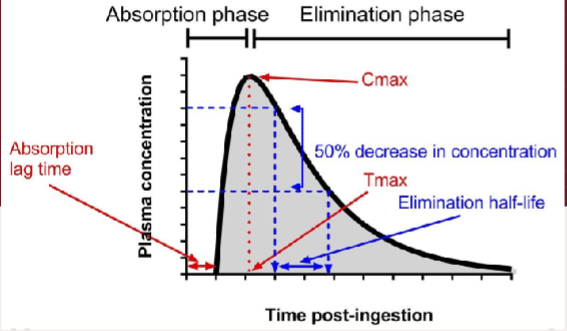
Plasma paracetamol levels can only be interpreted if blood is taken within how many hours of ingestion
4
(check units & time of ingestion carefully when using a graph!)
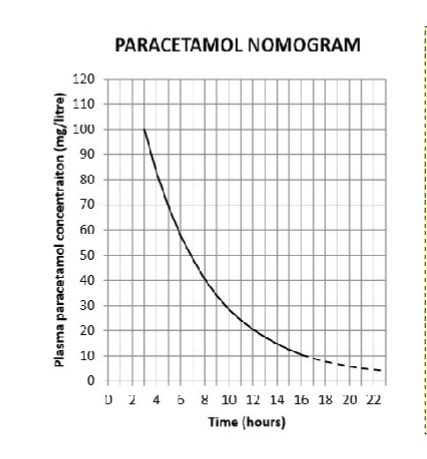
For what kind of overdose would this graph not be used
A staggered overdose
Distribution is used to calculate what in what phase of trials
Calculation of a non-toxic dose for clinical trials
(Preclinical and Phase 1)
How would distribution info be used to optimise Vd
Introduction of e.g. basic group to drug to optimise Vd
True/False: an ideal drug would be exclusively metabolised by a CYP450 enzyme
False: Ideal drug would not exclusively metabolised by a CYP450 enzyme (as there are lots of polymorphisms)
The enzyme system responsible for the metabolism of most drugs is:
A. P-glycoprotein.
B. alkaline phosphatase.
C. creatine kinase.
D. cytochrome P-450.
E. HMG-CoA.
D. cytochrome P-450
Elimination section next……..
What are the major(4) & minor(2) routes of drug excretion
• Renal
• Biliary / GI
• Pulmonary
• Skin
Minor: Mammary (delivery to baby!) & Salivary (therapeutic drug monitoring)
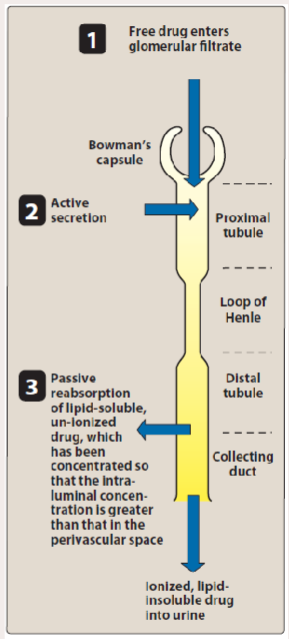
3 processes involved in renal elimination
• Glomerular Filtation
• Active Tubular Secretion
• Passive Reabsorption across Tubular Epithelium
Molecules less than __kDa are filtered (enter filtrate) in glomerular filtration
20kDa
Why kind of molecules don’t get filtered in glomerular filtration
Protein bound drugs not filtered (plasma albumin = 68kDa)
How does tubular secretion work
Active carrier-mediated elimination
Can transport against electrochemical gradient and when drug protein bound
How does renal reabsorption work
Passive diffusion back across tubular epithelium
What happens to lipid soluble drugs in renal reabsorption?
Lipid-soluble drugs have high tubular permeability, meaning they easily cross cell membranes.
Because of this, after being filtered into the tubule, they tend to diffuse back into the blood rather than stay in the urine.
As a result, they are excreted slowly in urine because much of the drug is reabsorbed into the bloodstream.
What type of molecules (polar/non-polar)(water/lipid soluble) remain in urine during renal reabsorption
polar water soluble drugs remain in urine
How does a drug ever find its way into renal filtrate
Free drug in plasma (unbound) diffuses across capillary membrane into filtrate in Bowman’s capsule
Are drugs affected by pH or lipid solubility when diffusing into the filtrate in Bowman’s capsule
No
Speed of movement into Bowman’s capsule (GFR)
120 mL/minute
What affects GFR
• Renal Blood Flow
• Glomerular Filtration Rate
• Drug Binding to Plasma Protein
What % of plasma doesn’t get filtered in the glomerulus
80%
How many carrier systems are there to transport drugs to the lumen
2 carrier systems transport drugs to lumen
• 1 carries acid drugs
• Other carries bases
How are drugs moved into the filtrate in renal tubules
Active Tubular Secretion against the electrochemical gradient
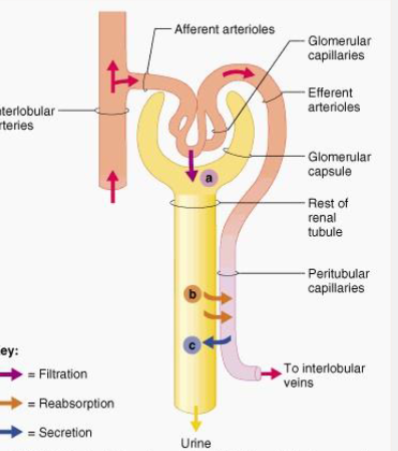
Name a drug that relies on being actively secreted into the renal tubules
Penicillin
True/False the active secretion of penicillin into the renal tubules lumen relies on plasma protein binding
False
We know that the active secretion of penicillin into the renal tubules lumen doesn’t rely on plasma protein binding, but what does it rely on
carrier proteins in the renal tubules specifically transport the free (unbound) drug from the plasma into the tubular fluid. (shift the equilibrium between bound and unbound drug)
Give an example of how competition can occur between drugs that use the same carrier system - use penicillin as an example
probenecid inhibits the tubular secretion of penicillin, thereby prolonging penicillin’s plasma concentration and therapeutic effect.
We know that water is greatly reabsorbed, but what about the filtered drug
99% of filtered drug is reabsorbed passively
What type of drugs are reabsorbed and what types stay in the lumen → excreted
Lipophilic drugs cross easily → reabsorbed
Polar (metabolized) drugs stay in lumen → Excreted