Gas Laws
0.0(0)
0.0(0)
Card Sorting
1/5
Earn XP
Description and Tags
final, chem 115, WVU fall '25
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
1
New cards
Dalton’s Law of Partial Pressures
in an ideal gas mixture, each gas exerts a pressure as if it’s alone in the mixture
2
New cards
Dalton’s Law
decreasing volume increases pressure
3
New cards
Avogadro’s Law
volume of gas is directly proportional to the number of moles
4
New cards
Charles’s Law
volume and temperature are proportional at constant pressure (doubling the temp of a gas doubles its volume)
5
New cards
Amonton’s Law
increasing temperature will increase pressure if volume is kept constant
6
New cards
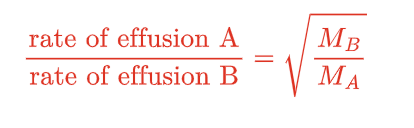
Graham’s Law of Effusion
rate of effusion of a gas is inversely proportional to the square root of its molar mass