SECTION 01: PROPERTIES OF AMINO ACIDS
1/195
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
196 Terms
What are amino acids?
Building blocks of proteins, essential for normal physiology.
What determines a protein’s folding and function?
The chemical nature of its amino acids.
Why study amino acids in depth?
To understand how their chemistry influences protein structure and function
What are the main interaction types between amino acids?
Noncovalent interactions (e.g. hydrogen bonds, ionic interactions, hydrophobic forces, Van der Waals).
What is the basic structure of an amino acid?
A: Central carbon (C) with:
Amino group (–NH₂)
Carboxyl group (–COOH)
Hydrogen atom
Unique R group (side chain)
What is a peptide bond?
A covalent bond between the carboxyl group of one amino acid and the amino group of another.
How are amino acids classified?
By their side chain properties:
Nonpolar (hydrophobic)
Polar uncharged
Acidic (negatively charged)
Basic (positively charged)
What are post-translational modifications (PTMs)?
Chemical changes to proteins after synthesis (e.g. phosphorylation, methylation) that affect protein function.
What are noncovalent interactions?
Weak, temporary bonds that can be broken and reformed easily.
Why are noncovalent bonds important in the body?
They help maintain structures and allow reversible interactions between molecules.
Example of noncovalent interaction in real life?
Human growth hormone (hGH) binding to its receptor.
How does hGH use noncovalent bonds?
A:
hGH binds to its receptor using noncovalent interactions.
This triggers cell growth and metabolism.
hGH then unbinds, stopping the signal.
What would happen if hGH binding was covalent (permanent)?
A: The hormone would stay stuck, causing constant growth — potentially dangerous and unregulated.
Why are noncovalent bonds better in this context?
A: They allow temporary, controlled signals instead of permanent effects.
What are the 4 main types of noncovalent interactions in biochemistry?
A:
Electrostatic (charge–charge)
Molecular dipoles
Hydrogen bonds
Hydrophobic/hydrophilic interactions
Are noncovalent interactions strong or weak?
Individually weak, but very strong when many act together (e.g., in a protein’s structure).
Why are noncovalent interactions important in proteins?
They help stabilize 3D structure and prevent denaturation (unfolding).
What is denaturation?
The unfolding or loss of 3D protein structure due to stress like heat or pH changes.
How many noncovalent interactions can be in a protein?
Hundreds to thousands, all working together to maintain structure and function.
What are electrostatic interactions?
Attractions between oppositely charged molecules (positive ↔ negative).
What are other names for electrostatic interactions?
A: Ionic interactions or salt bridges.
Where do electrostatic interactions happen in the body?
In many places—proteins, DNA, and between molecules that carry charges.
Example of electrostatic interaction in the cell?
A:
DNA = negatively charged
DNA-binding proteins = have positively charged regions
→ These opposite charges attract, allowing the protein to bind to DNA.
Why are electrostatic interactions important?
They help molecules stick together temporarily, supporting functions like DNA replication
What is a molecular dipole?
When an uncharged molecule has an uneven distribution of electrons, making one end slightly positive (δ⁺) and the other slightly negative (δ⁻).
Example of a molecule with a dipole?
A: Carbon monoxide (CO):
Oxygen pulls electrons → δ⁻
Carbon loses electrons → δ⁺
What is a dipole moment?
A measure of polarity—how unevenly charges are shared in a bond.
How do dipoles interact with each other?
Molecules align opposite each other (δ⁺ facing δ⁻) to maximize attraction.
Why are dipole interactions important?
They help molecules stick together and influence structure and function without full charges being present.
What are polar molecules?
Molecules with strong dipole moments due to uneven electron distribution.
What makes a molecule polar?
A:
Electron-withdrawing atoms (O, N, S, P)
Asymmetrical shape that prevents dipoles from canceling out
Is water polar? Why?
A: Yes—oxygen pulls electrons away from hydrogen, creating a bent shape with a strong dipole.
Is CO₂ polar? Why or why not?
No—symmetrical linear shape causes dipoles to cancel out, so there’s no overall dipole moment.
What’s a quick tip to identify polar molecules?
A: Look for O, N, S, or P atoms and asymmetry in shape or charge distribution.
What is a hydrogen bond?
A: A strong noncovalent interaction between a slightly positive hydrogen atom and a highly electronegative atom (like O, N, or S) with a lone pair of electrons.
Why are hydrogen bonds important in biochemistry?
A: They help shape proteins and DNA, affecting how they fold, function, and interact.
What are the two parts of a hydrogen bond?
A:
Hydrogen Donor: The atom (O, N, or S) bonded to H, creating a partial positive charge on H
Hydrogen Acceptor: The electronegative atom (usually O or N) with a lone electron pair that attracts the hydrogen
Why is the hydrogen slightly positive in a hydrogen bond?
A: Because electronegative atoms like oxygen or nitrogen pull electrons away from hydrogen, leaving it with a δ⁺ (partial positive) charge.
What atoms can form hydrogen bonds?
Nitrogen (N), Oxygen (O), Sulphur (S) – all have high electronegativity and lone pairs.
: Are hydrogen bonds real bonds?
A: No – they aren’t true covalent bonds, but they are stronger than most other noncovalent interactions.
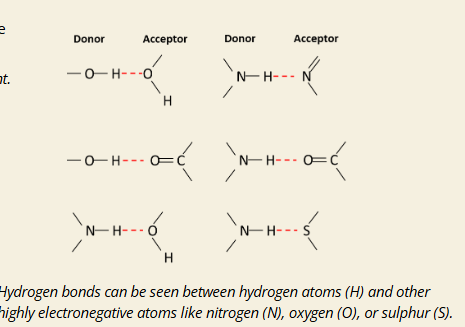
Q: Example from image: What’s happening in the H-bond between two water molecules?
A: One O-H group donates a hydrogen, and the oxygen from another water molecule accepts it via its lone pair → forming an H-bond.
Q: What’s a dipole moment?
A: A measure of how unevenly electrons are shared in a bond. Bigger dipole = stronger pull toward one atom.
What do hydrophobic and hydrophilic mean?
Hydrophobic = “Water-fearing” → doesn’t mix with water
Hydrophilic = “Water-loving” → mixes well with water
What is a phospholipid?
A: A molecule with both hydrophobic and hydrophilic parts (called amphipathic).
What part of the phospholipid is hydrophilic?
A: The head — it’s polar, so it likes water and faces outward toward watery environments (inside and outside the cell).
What part of the phospholipid is hydrophobic?
A: The tail — made of nonpolar hydrocarbons, so it hates water and points inward, away from water.
Q: What happens when phospholipids are in water?
A: They self-organize into a bilayer:
Heads face water
Tails face each other → forming the cell membrane
What is the hydrophobic effect?
A: Water forms an ordered shell around hydrophobic molecules, pushing them together to minimize disruption → this drives the membrane to form.
What are examples of hydrophilic molecules?
A: Ionic and polar molecules (e.g., Na⁺, Cl⁻, water-soluble vitamins)
Q: What are examples of hydrophobic molecules?
A: Hydrocarbons in rings (aromatic) or chains (aliphatic), like fats and oils.
Q: What is the hydrophobic effect?
A: The tendency of nonpolar molecules to clump together in water to reduce disruption to water’s structure.
What causes the hydrophobic effect?
A: Entropy (disorder). Water becomes more disordered when nonpolar molecules group together — and nature favors disorder!
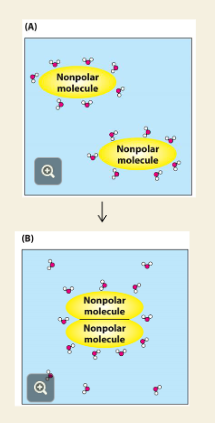
Q: What happens in (A) of the image?
A: Nonpolar molecules are separate, and water forms ordered cages around each — this decreases entropy (bad for the system).
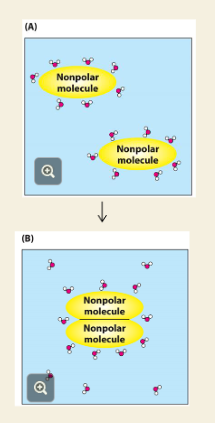
Q: What happens in (B) of the image?
A: Nonpolar molecules group together, so less water has to be ordered around them → entropy increases (more free water = good).
Q: Do nonpolar molecules "like" each other?
A: No — they don't attract. They just stick together to avoid disrupting water.
Why is the hydrophobic effect important?
A: It's crucial for:
Cell membrane formation (phospholipids)
Protein folding (hydrophobic parts fold inside)
Q: What law explains this effect?
A: The 2nd Law of Thermodynamics — systems move toward greater entropy (disorder).

Activity
What is an amino acid?
A: A small organic molecule that is the building block of proteins.
Q: How many amino acids are used in human protein synthesis?
A: 20 amino acids out of over 300 found in nature.
Q: What are the 4 parts attached to the central (alpha) carbon in an amino acid?
A:
Amino group (–NH₂)
Carboxyl group (–COOH)
Hydrogen atom (–H)
Side chain (R-group) → varies for each amino acid
Q: What is the α-carbon (Cα)?
A: The central carbon in every amino acid — all groups attach to it.
Q: What is the α-amino group?
A: The –NH₂ group attached to the α-carbon; it can carry extra hydrogens depending on pH or bonding.
Q: What is the α-carboxyl group?
A: The –COOH group attached to the α-carbon; it can lose a hydrogen and become negatively charged at physiological pH.
Q: What is the R-group (side chain)?
A: The unique part of each amino acid that determines its properties and behavior (e.g., polar, nonpolar, acidic, basic).
Q: Why is the R-group important?
A: It affects how the amino acid interacts, folds, and functions in a protein.
Q: What is a protein made of?
A: A polymer (long chain) of amino acids linked together like beads on a string.
Q: What holds amino acids together in a protein chain?
A: Peptide bonds — strong covalent bonds between the carboxyl group of one amino acid and the amino group of another.
Q: What determines how a protein folds?
A: R-group interactions with each other and with the environment (e.g., water, pH, temperature).
Q: Why is protein folding important?
A: The 3D structure created by folding determines the function of the protein.
Q: What is myoglobin?
A: A protein found in muscle that binds oxygen — shown as an example of a folded protein.
Q: What are different ways to view protein structures?
A:
Ball-and-stick model
Ribbon diagram
Space-filling model
Each shows different levels of detail.
Q: What is a polymer?
A: A large molecule made of repeating subunits (like amino acids in a protein).
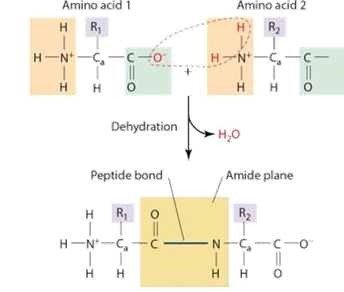
Q: What is a peptide bond?
A: A covalent bond that links the carboxyl group of one amino acid to the amino group of another.
Q: How does a peptide bond form?
A: Through a condensation reaction — a water molecule (H₂O) is removed when the bond forms.
Q: What kind of reaction breaks a peptide bond?
A: Hydrolysis — water is added back to break the bond.
Q: Are peptide bonds stable?
A: Yes — they are very strong and rarely break without help.
Q: What helps break peptide bonds in the body?
A: Enzymes like peptidases or proteases — they catalyze the hydrolysis of peptide bonds.
Q: What happens to the atoms during peptide bond formation?
A: The OH from the carboxyl group and an H from the amino group combine to form H₂O, leaving a new C–N bond behind.
Q: What is the amide plane?
A: The flat region created by the peptide bond, due to partial double-bond character between the C=O and N–H.
Q: How are the 20 amino acids classified?
A: Into 5 groups based on R-group (side chain) chemistry.
Q: Why are R-groups important?
A: They determine each amino acid’s properties and how they interact in proteins.
Q: What are the 5 amino acid categories?
A:
Nonpolar
Uncharged polar
Acidic (negatively charged)
Basic (positively charged)
Aromatic
Q: What are nonpolar amino acids like?
A: Hydrophobic, don’t interact well with water → tend to be buried inside proteins.
Q: What are uncharged polar amino acids like?
A: Hydrophilic, form hydrogen bonds, often on the outside of proteins.
Q: What are acidic amino acids like?
A: Have negatively charged R-groups at physiological pH (e.g., aspartic acid, glutamic acid).
Q: What are basic amino acids like?
A: Have positively charged R-groups (e.g., lysine, arginine, histidine).
Q: What are aromatic amino acids like?
A: Have ring-shaped R-groups that can absorb UV light and are often involved in stacking interactions (e.g., phenylalanine, tyrosine, tryptophan).
Q: What do you need to memorize for this course?
A: For all 20 amino acids:
Full name
3-letter code
1-letter code
Structure
Properties
Q: What are nonpolar side chains?
A: Amino acid side chains that are hydrophobic and don’t interact well with water.
Q: Can nonpolar side chains gain/lose protons or form hydrogen/ionic bonds?
A: No — they cannot form H-bonds or ionic bonds and don’t carry charge.
Q: What happens to nonpolar amino acids in water?
A: They cluster together to avoid water — this is part of the hydrophobic effect.
Q: Where are nonpolar amino acids found in soluble (water-based) proteins?
A: In the core of the protein, hidden away from water.
🟡 Represented by yellow circles in the image.
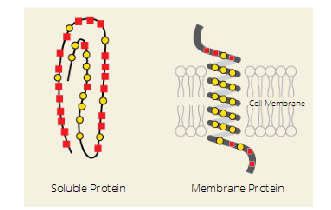
Q: Where are nonpolar amino acids found in membrane proteins?
A: They are on the outside, interacting with the hydrophobic lipid tails of the membrane.
Q: What are the red squares in the image?
A: Hydrophilic amino acids — these face water in both soluble and membrane proteins.
🟥 Represented by red squares in the image.
Q: What is the main role of nonpolar side chains in proteins?
A: They drive protein folding by grouping together away from water, stabilizing the protein structure.
Q: What defines a nonpolar amino acid?
A: Its R-group is hydrophobic (mostly C and H) → avoids water, found inside proteins or in lipid membranes.
What are the 9 nonpolar amino acids?
A:
Glycine (Gly, G)
Alanine (Ala, A)
Valine (Val, V)
Leucine (Leu, L)
Isoleucine (Ile, I)
Methionine (Met, M)
Proline (Pro, P)
Phenylalanine (Phe, F)
Tryptophan (Trp, W)
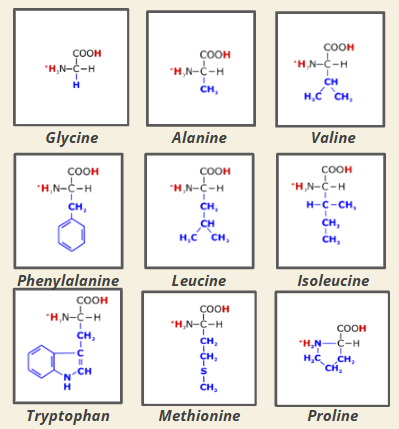
Q: What’s special about Glycine?
A: Smallest amino acid – R-group is just H → adds flexibility to proteins.
Q: What do Alanine, Valine, Leucine, and Isoleucine have in common?
A: Their side chains are hydrocarbons (C and H only).
Alanine → CH₃
Valine → branched CH(CH₃)₂
Leucine → longer branch
Isoleucine → similar to leucine but different shape